Acid-Stable Cu Cluster Precatalysts Enable High Energy and Carbon Efficiency in CO2 Electroreduction
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical reduction of CO2 in acidic media offers the advantage of high carbon utilization, but achieving high selectivity to C2+ products at a low overpotential remains a challenge. We identified the chemical instability of oxide-derived Cu catalysts as a reason that advances in neutral/alkaline electrolysis do not translate to acidic conditions. In acid, Cu ions leach from Cu oxides, leading to the deactivation of the C2+-active sites of Cu nanoparticles. This prompted us to design acid-stable Cu cluster precatalysts that are reduced in situ to active Cu nanoparticles in strong acid. Operando Raman and X-ray spectroscopy indicated that the bonding between the Cu cluster precatalyst ligand and in situ formed Cu nanoparticles preserves a high density of undercoordinated Cu sites, resulting in a C2H4 Faradaic efficiency of 62% at a low overpotential. The result is a 1.4-fold increase in energy efficiency compared with previous acidic CO2-to-C2+ electrocatalytic systems.
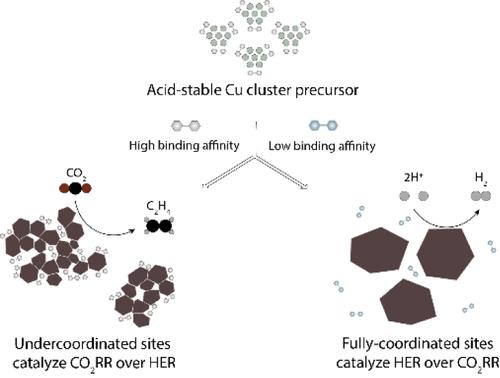
酸稳定性铜簇前催化剂可在二氧化碳电还原过程中实现高能量和高碳效率
在酸性介质中电化学还原二氧化碳具有碳利用率高的优势,但在低过电位条件下实现对 C2+ 产物的高选择性仍然是一项挑战。我们发现,氧化物衍生的 Cu 催化剂化学性质不稳定,这是中性/碱性电解技术的进步无法应用于酸性条件的原因。在酸性条件下,铜离子从铜氧化物中渗出,导致铜纳米颗粒的 C2+ 活性位点失活。这促使我们设计出酸性稳定的铜簇前催化剂,这种催化剂可在强酸中就地还原成活性铜纳米粒子。操作拉曼光谱和 X 射线光谱表明,铜簇前催化剂配体与原位形成的铜纳米粒子之间的键合保留了高密度的欠配位铜位点,从而在低过电位下实现了 62% 的 C2H4 法拉第效率。与之前的酸性 CO2-C2+ 电催化系统相比,能量效率提高了 1.4 倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


