Photodriven Ammonia Synthesis from Manganese Nitrides: Photophysics and Mechanistic Investigations
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ammonia synthesis from N,N,O,O-supported manganese(V) nitrides and 9,10-dihydroacridine using proton-coupled electron transfer and visible light irradiation in the absence of precious metal photocatalysts is described. While the reactivity of the nitride correlated with increased absorption of blue light, excited-state lifetimes determined by transient absorption were on the order of picoseconds. This eliminated excited-state manganese nitrides as responsible for bimolecular N–H bond formation. Spectroscopic measurements on the hydrogen source, dihydroacridine, demonstrated that photooxidation of 9,10-dihydroacridine was necessary for productive ammonia synthesis. Transient absorption and pulse radiolysis data for dihydroacridine provided evidence for the presence of intermediates with weak E–H bonds, including the dihydroacridinium radical cation and both isomers of the monohydroacridine radical, but notably these intermediates were unreactive toward hydrogen atom transfer and net N–H bond formation. Additional optimization of the reaction conditions using higher photon flux resulted in higher rates of the ammonia production from the manganese(V) nitrides due to increased activation of the dihydroacridine.
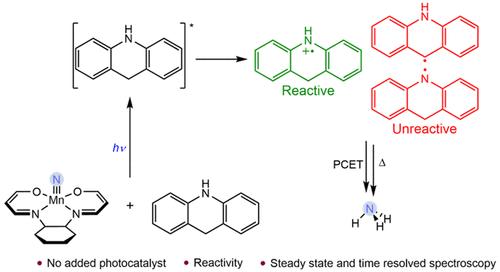
氮化锰的光驱动氨合成:光物理学和机理研究
本研究描述了在没有贵金属光催化剂的情况下,利用质子耦合电子转移和可见光照射,从 N,N,O,O-支撑的氮化锰(V)和 9,10-二氢吖啶中合成氨的过程。虽然氮化物的反应活性与对蓝光吸收的增加有关,但通过瞬态吸收确定的激发态寿命为皮秒量级。这就排除了激发态氮化锰形成双分子 N-H 键的可能性。对氢源二氢吖啶的光谱测量表明,9,10-二氢吖啶的光氧化作用是合成氨的必要条件。二氢吖啶的瞬态吸收和脉冲辐射数据证明了存在弱 E-H 键的中间产物,包括二氢吖啶自由基阳离子和单氢吖啶自由基的两种异构体,但值得注意的是,这些中间产物对氢原子转移和净 N-H 键的形成没有反应。使用更高的光子通量对反应条件进行进一步优化后,由于二氢吖啶的活化程度提高,氮化锰(V)的氨生产率也随之提高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


