Ferrocene- and ruthenium arene-containing glycomimetics as selective inhibitors of human galectin-1 and -3
IF 6.1
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Galectins are a family of β-galactoside-binding proteins with an evolutionarily conserved carbohydrate recognition domain. Their dysregulation has been implicated in physiological and pathological processes, including fibrotic disorders, inflammation, and cancer. For example, elevated levels of galectin-1 contribute to tumor cell migration and immune evasion, whereas overexpression of galectin-3 is associated with increased invasiveness and the formation of metastasis. Pharmacological inhibition of these galectins is a promising therapeutic strategy to counteract their oncogenic effects. In this study, we synthesized a novel series of galectin inhibitors with ferrocene and ruthenium arene motifs attached to lactose, N-acetyllactosamine, or thiodigalactoside scaffolds. We determined their binding affinity toward human galectin-1 (hgal-1) and the CRD domain of human galectin-3 (hgal-3-CRD) using fluorescence polarization, intrinsic fluorescence of galectin tryptophan residues, and isothermal titration calorimetry. The ferrocene analogs exhibited superior affinity for both hgal-1 and hgal-3-CRD compared with ruthenium arenes. In particular, a symmetrical diferrocene thiodigalactoside complex exhibited low nanomolar affinity for hgal-1 and selectivity over hgal-3-CRD. Asymmetrical monoferrocene thiodigalactoside complexes exhibited nanomolar affinity and good selectivity for hgal-3-CRD. Chronopotentiometric stripping analysis demonstrated that the inhibitors stabilized hgal-1 against destabilization by electric field effects. 19F{1H} NMR experiments and molecular dynamics simulations suggested that the incorporation of the ferrocene motif limited the accessible binding modes to hgal-3-CRD whereas binding to hgal-1 remained unrestricted, resulting in attenuated binding affinities to hgal-3-CRD and selectivity for hgal-1. These results open new possibilities for the design and optimization of therapeutic organometallic galectin inhibitors.
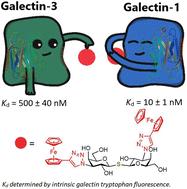
含二茂铁和钌炔的拟糖化物作为人类 galectin-1 和 -3 的选择性抑制剂
半乳糖苷结合蛋白(Galectins)是一个具有进化保守的碳水化合物识别结构域的β-半乳糖苷结合蛋白家族。它们的失调与生理和病理过程有关,包括纤维化疾病、炎症和癌症。例如,galectin-1 水平的升高有助于肿瘤细胞的迁移和免疫逃避,而 galectin-3 的过度表达则与侵袭性增加和转移的形成有关。药理抑制这些galectin是对抗其致癌作用的一种有前景的治疗策略。在这项研究中,我们合成了一系列新型的galectin抑制剂,这些抑制剂的二茂铁和钌炔基团附着在乳糖、N-乙酰半乳糖胺或硫代二半乳糖苷支架上。我们利用荧光偏振、galectin 色氨酸残基的本征荧光和等温滴定量热法测定了它们与人 galectin-1(hgal-1)和人 galectin-3 的 CRD 结构域(hgal-3-CRD)的结合亲和力。与钌烷相比,二茂铁类似物对 hgal-1 和 hgal-3-CRD 的亲和力更强。尤其是对称二茂铁硫代半乳糖苷复合物对 hgal-1 的亲和力低至纳摩尔,对 hgal-3-CRD 的选择性更高。不对称的单二茂铁硫代二半乳糖苷复合物对 hgal-3-CRD 具有纳摩尔亲和力和良好的选择性。计时电位剥离分析表明,抑制剂能稳定 hgal-1,防止其因电场效应而失稳。19F{1H}核磁共振实验和分子动力学模拟表明,二茂铁基团的加入限制了与 hgal-3-CRD 的结合模式,而与 hgal-1 的结合则不受限制,从而削弱了与 hgal-3-CRD 的结合亲和力以及对 hgal-1 的选择性。这些结果为设计和优化治疗性有机金属 galectin 抑制剂提供了新的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry Frontiers
CHEMISTRY, INORGANIC & NUCLEAR-
CiteScore
10.40
自引率
7.10%
发文量
587
审稿时长
1.2 months
期刊介绍:
The international, high quality journal for interdisciplinary research between inorganic chemistry and related subjects
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


