First-principles study of efficient integral water-splitting and oxygen reduction reactions in transition metal single atom anchored NbTe2
IF 3.1
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite the tendency of single-atom catalysts (SACs) to form nanoclusters due to high surface free energy, which reduces their catalytic activity, the high atomic utilization rate and low cost of SACs continue to make them a current research focus. Herein, the first-principles calculations are employed to design transition metal-doped NbTe2 single-atom catalysts (TM@NbTe2) for water-splitting reactions. The strong chemical bonds formed between the transition metal (TM) atoms and the NbTe2 enhance catalytic activity and stability. By calculating the free energies of intermediates, Sc@NbTe2 and V@NbTe2 exhibited HER overpotentials of 0.086 V and 0.238 V, respectively, representing reductions of about 93 % and 80 % compared to the original NbTe2. When studying OER performance, the optimized model TM@NbTe2 was used to analyze and calculate the electron transfer and redistribution in the four-electron OER process, Ni@NbTe2 and Fe@NbTe2 had overpotentials of 0.36 V and 0.49 V, respectively, which are reductions of about 86 % and 81 % compared to the original NbTe2. Simultaneously, both exhibited significant ORR activity. Surprisingly, Fe@NbTe2 is considered to have the potential to become a trifunctional catalyst for HER, OER, and ORR. This study establishes a theoretical bridge towards the practical application of highly efficient NbTe2-based water electrolysis catalysts.
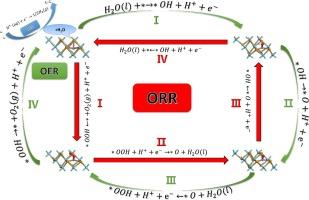
过渡金属单原子锚定 NbTe2 中高效整体分水和氧还原反应的第一性原理研究
尽管单原子催化剂(SAC)由于表面自由能较高而容易形成纳米团簇,从而降低了其催化活性,但其原子利用率高、成本低的特点仍然使其成为当前的研究热点。本文采用第一性原理计算设计了用于分水反应的过渡金属掺杂 NbTe2 单原子催化剂(TM@NbTe2)。过渡金属 (TM) 原子与 NbTe2 之间形成的强化学键提高了催化活性和稳定性。通过计算中间产物的自由能,Sc@NbTe2 和 V@NbTe2 的 HER 过电位分别为 0.086 V 和 0.238 V,与原始 NbTe2 相比分别降低了约 93% 和 80%。在研究OER性能时,使用优化模型TM@NbTe2来分析和计算四电子OER过程中的电子转移和再分配,Ni@NbTe2和Fe@NbTe2的过电位分别为0.36 V和0.49 V,与原始NbTe2相比分别降低了约86%和81%。同时,两者都表现出了显著的 ORR 活性。令人惊讶的是,Fe@NbTe2 被认为具有成为 HER、OER 和 ORR 三功能催化剂的潜力。这项研究为基于 NbTe2 的高效水电解催化剂的实际应用搭建了一座理论桥梁。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational Materials Science
工程技术-材料科学:综合
CiteScore
6.50
自引率
6.10%
发文量
665
审稿时长
26 days
期刊介绍:
The goal of Computational Materials Science is to report on results that provide new or unique insights into, or significantly expand our understanding of, the properties of materials or phenomena associated with their design, synthesis, processing, characterization, and utilization. To be relevant to the journal, the results should be applied or applicable to specific material systems that are discussed within the submission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


