Structural insights into the initiation of free radical formation in the Class Ib ribonucleotide reductases in Mycobacteria
IF 2.7
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
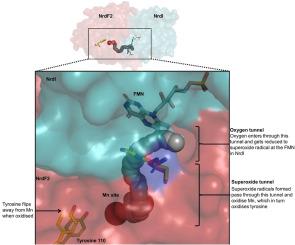
Class I ribonucleotide reductases consisting of α and β subunits convert ribonucleoside diphosphates to deoxyribonucleoside diphosphates involving an intricate free radical mechanism. The generation of free radicals in the Class Ib ribonucleotide reductases is mediated by di-manganese ions in the β subunits and is externally assisted by flavodoxin-like NrdI subunit. This is unlike Class Ia ribonucleotide reductases, where the free radical generation is initiated at its di-iron centre in the β subunits with no external support from another subunit. Class 1b ribonucleotide reductase complex is an essential enzyme complex in the human pathogen Mycobacterium tuberculosis and its structural details are largely unknown. In this study we have determined the crystal structures of Mycobacterial NrdI in oxidised and reduced forms, and similarly those of NrdF2:NrdI complexes. These structures provide detailed atomic view of the mechanism of free radical generation in the β subunit in this pathogen. We observe a well-formed channel in NrdI from the surface leading to the buried FMN moiety and propose that oxygen molecule accesses FMN through it. The oxygen molecule is further converted to a superoxide ion upon electron transfer at the FMN moiety. Similarly, a path for superoxide radical transfer between NrdI and NrdF2 is also observed. The oxidation of Mn(II) in NrdF2I to high valent oxidation state (either Mn(III) or Mn(IV) assisted by the reduced FMN site was evidently confirmed by EPR studies. SEC-MALS and low resolution cryo-EM map indicate unusual stoichiometry of 2:1 in the M. tuberculosis NrdF2I complex. A density close to Tyr 110 at a distance <2.3 Å is observed, which we interpret as OH group. Overall, the study therefore provides important clues on the initiation of free radical generation in the β subunit of the ribonucleotide reductase complex in M. tuberculosis.
分枝杆菌 Ib 类核糖核苷酸还原酶自由基形成的结构启示
由 α 和 β 亚基组成的 I 类核糖核苷酸还原酶通过复杂的自由基机制将核糖核苷二磷酸转化为脱氧核糖核苷二磷酸。Ib 类核糖核苷酸还原酶中自由基的生成由 β 亚基中的二锰离子介导,并由类似黄酮甙的 NrdI 亚基提供外部协助。这与 Ia 类核糖核苷酸还原酶不同,后者的自由基生成是由β亚基中的二铁中心启动的,没有其他亚基的外部支持。1b 类核糖核苷酸还原酶复合物是人类病原体结核分枝杆菌中一种重要的酶复合物,其结构细节大多不为人知。在这项研究中,我们测定了分枝杆菌 NrdI 氧化型和还原型的晶体结构,以及 NrdF2:NrdI 复合物的晶体结构。这些结构提供了该病原体中 β 亚基产生自由基机制的详细原子视图。我们在 NrdI 中观察到一个形成良好的通道,从表面通向埋藏的 FMN 分子,并提出氧分子通过该通道进入 FMN。氧分子在 FMN 分子上进行电子转移后进一步转化为超氧离子。同样,在 NrdI 和 NrdF2 之间也观察到超氧自由基转移的路径。在还原型 FMN 位点的协助下,NrdF2I 中的锰(II)被氧化为高价氧化态(锰(III)或锰(IV)),这一点已被 EPR 研究明显证实。SEC-MALS 和低分辨率低温电子显微镜图显示,结核杆菌 NrdF2I 复合物中的化学计量为 2:1。在距离 Tyr 110 <2.3 Å 处观察到一个密度,我们将其解释为 OH 基团。总之,这项研究为结核杆菌核糖核苷酸还原酶复合物β亚基中自由基的生成提供了重要线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


