Altered copper transport in oxidative stress-dependent brain endothelial barrier dysfunction associated with Alzheimer's disease
IF 3.5
3区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
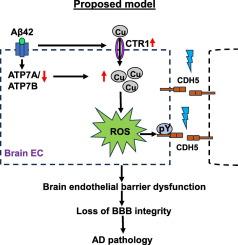
Oxidative stress and blood-brain barrier (BBB) disruption due to brain endothelial barrier dysfunction contribute to Alzheimer's Disease (AD), which is characterized by beta-amyloid (Aβ) accumulation in senile plaques. Copper (Cu) is implicated in AD pathology and its levels are tightly controlled by several Cu transport proteins. However, their expression and role in AD, particularly in relation to brain endothelial barrier function remains unclear. In this study, we examined the expression of Cu transport proteins in the brains of AD mouse models as well as their involvement in Aβ42-induced brain endothelial barrier dysfunction. We found that the Cu uptake transporter CTR1 was upregulated, while the Cu exporter ATP7A was downregulated in the hippocampus of AD mouse models and in Aβ42-treated human brain microvascular endothelial cells (hBMECs). In the 5xFAD AD mouse model, Cu levels (assessed by ICP-MS) were elevated in the hippocampus. Moreover, in cultured hBMECs, Aβ42-induced reactive oxygen species (ROS) production, ROS-dependent loss in barrier function (measured by transendothelial electrical resistance), and tyrosine phosphorylation of CDH5 were all inhibited by either a membrane permeable Cu chelator or by knocking down CTR1 expression. These findings suggest that dysregulated expression of Cu transport proteins may lead to intracellular Cu accumulation in the AD brain, and that Aβ42 promotes ROS-dependent brain endothelial barrier dysfunction and CDH5 phosphorylation in a CTR1-Cu-dependent manner. Our study uncovers the critical role of Cu transport proteins in oxidative stress-related loss of BBB integrity in AD.
与阿尔茨海默病相关的氧化应激依赖性脑内皮屏障功能障碍中的铜转运改变
氧化应激和脑内皮屏障功能障碍导致的血脑屏障(BBB)破坏是阿尔茨海默病(AD)的诱因,阿尔茨海默病的特征是老年斑中β-淀粉样蛋白(Aβ)的积累。铜(Cu)与阿尔兹海默病的病理有关,其含量受到几种铜转运蛋白的严格控制。然而,它们在老年痴呆症中的表达和作用,尤其是与脑血管内皮屏障功能的关系仍不清楚。在这项研究中,我们检测了AD小鼠模型脑中铜转运蛋白的表达及其在Aβ42诱导的脑血管内皮屏障功能障碍中的参与情况。我们发现,在AD小鼠模型的海马和Aβ42处理的人脑微血管内皮细胞(hBMECs)中,铜吸收转运蛋白CTR1上调,而铜输出蛋白ATP7A下调。在 5xFAD AD 小鼠模型中,海马中的铜含量(通过 ICP-MS 评估)升高。此外,在培养的 hBMECs 中,Aβ42 诱导的活性氧(ROS)产生、ROS 依赖性屏障功能丧失(通过跨内皮电阻测量)以及 CDH5 的酪氨酸磷酸化均受到膜渗透性铜螯合剂或 CTR1 表达基因敲除的抑制。这些研究结果表明,铜转运蛋白表达失调可能会导致AD大脑细胞内的铜积累,而Aβ42会以CTR1-Cu依赖的方式促进ROS依赖的脑内皮屏障功能障碍和CDH5磷酸化。我们的研究揭示了铜转运蛋白在与氧化应激相关的AD脑血管屏障完整性丧失中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Vascular pharmacology
医学-药学
CiteScore
6.60
自引率
2.50%
发文量
153
审稿时长
31 days
期刊介绍:
Vascular Pharmacology publishes papers, which contains results of all aspects of biology and pharmacology of the vascular system.
Papers are encouraged in basic, translational and clinical aspects of Vascular Biology and Pharmacology, utilizing approaches ranging from molecular biology to integrative physiology. All papers are in English.
The Journal publishes review articles which include vascular aspects of thrombosis, inflammation, cell signalling, atherosclerosis, and lipid metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


