Photoinduced Nickel-Catalyzed Homolytic C(sp3)–N Bond Activation of Isonitriles for Selective Carbo- and Hydro-Cyanation of Alkynes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The exploration of strong chemical bonds as synthetic handles offers new disconnection strategies for the synthesis of functionalized molecules via transition metal catalysis. However, the slow oxidative addition rate of these covalent bonds to a transition metal center hampers their synthetic utility. Here, we report a C(sp3)–N bond activation strategy that bypasses thermodynamically challenging 2e– or 1e– oxidative addition via a distinct pathway in nickel catalysis. This strategy leverages a previously unknown activation pathway of photoinduced inner-sphere charge transfer of low-valent nickel(isonitriles), triggering a C(sp3)–N bond cleavage distal to the metal–ligand interaction to deliver nickel(cyanide) and versatile alkyl radicals. Utilizing this catalytic strategy, the selective intermolecular 1,2-carbocyanation reaction of alkynes with alkyl isonitriles as both alkylating and cyanating agents can be achieved, delivering a wide array of trisubstituted alkenyl nitriles with excellent atom-economy, regio-, and stereoselectivity under mild conditions. Furthermore, Markovnikov-selective hydrocyanation of aliphatic alkynes can be accomplished through the synergistic action of a photocatalyst utilizing isonitriles as the cyanation agents. Mechanistic investigations support the photogeneration of low-valent Ni(isonitrile) complexes that undergo photochemical homolysis of the C(sp3)–N bond to engage catalytic cyanation with alkynes.
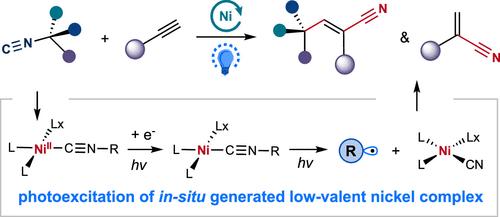
光诱导镍催化异腈的 C(sp3)-N 键同源分解活化,用于炔烃的选择性碳酸化和氢氰化
将强化学键作为合成工具的探索为通过过渡金属催化合成功能化分子提供了新的断开策略。然而,这些共价键与过渡金属中心的缓慢氧化加成速率阻碍了它们的合成用途。在此,我们报告了一种 C(sp3)-N 键活化策略,它通过镍催化的独特途径绕过了热力学上具有挑战性的 2e- 或 1e- 氧化加成。该策略利用了低价镍(异腈)的光诱导内圈电荷转移这一先前未知的活化途径,在金属-配体相互作用的远端引发 C(sp3)-N 键裂解,从而产生镍(氰化物)和多功能烷基自由基。利用这种催化策略,可以实现以烷基异腈为烷化剂和氰化剂的炔烃分子间选择性 1,2-羰基氰化反应,在温和的条件下生成一系列具有优异原子经济性、区域选择性和立体选择性的三取代烯基腈化物。此外,通过利用异腈作为氰化剂的光催化剂的协同作用,可以实现脂肪族炔烃的马尔科夫尼科夫选择性氢氰化反应。机理研究支持低价镍(异腈)络合物的光生成,这种络合物会对 C(sp3)-N 键进行光化学均解,从而与炔烃发生催化氰化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


