What is the true ground state of intermetallic compound Fe3Al?
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
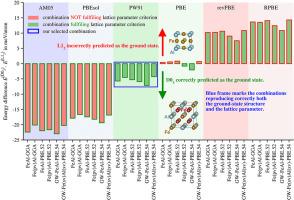
We discuss recent doubts about the true ground-state (GS) structure of the intermetallic compound Fe3Al. It seems that it should be the D03 structure (observed experimentally), but there are some considerations that, perhaps, D03 might be a high-temperature (>400 K) structure and the GS at 0 K might be the L12 structure because there might be a high energy barrier between both structures and, when the temperature is lowered, the system is not able to transform into the (perhaps) lower-energy L12 structure. To elucidate this problem, we re-interpret our recent extended ab initio electronic structure calculations for Fe3Al performed with the help of the VASP code and using various exchange-correlation energies within the generalized gradient approximation (GGA). Regrettably, some calculations provide the L12 and some of them D03 as the GS structure.
To resolve this question, we performed further calculations testing 9 frequently applied metaGGAs, such as TPSS, revTPSS, M06-L, SCAN(-L), rSCAN(-L) and r2SCAN(-L) representing a higher rung of the Jacob's ladder. It turns out that also here some meta-GGAs lead to L12 and some others to D03 GS structure and, again, we cannot decide.
In this way, the present results represent the very first step on the way to understand the energetics of the Fe3Al compound and its ground state. We hope they may motivate future theoretical and experimental work in this direction.
金属间化合物 Fe3Al 的真实基态是什么?
我们讨论了最近对金属间化合物 Fe3Al 的真实基态(GS)结构的怀疑。它似乎应该是 D03 结构(实验观察到的),但也有人认为,D03 可能是一种高温(400 K)结构,而 0 K 时的 GS 可能是 L12 结构,因为这两种结构之间可能存在高能量屏障,当温度降低时,系统无法转变为(可能)能量较低的 L12 结构。为了阐明这个问题,我们重新解释了最近在 VASP 代码的帮助下,利用广义梯度近似(GGA)中的各种交换相关能量对 Fe3Al 进行的扩展 ab initio 电子结构计算。为了解决这个问题,我们进行了进一步的计算,测试了 9 种常用的元 GGA,如 TPSS、revTPSS、M06-L、SCAN(-L)、rSCAN(-L) 和 r2SCAN(-L),它们代表了雅各布阶梯的更高一级。事实证明,同样在这里,一些元 GGA 导致了 L12,而另一些则导致了 D03 GS 结构,我们同样无法做出决定。我们希望这些结果能激励我们今后朝着这个方向开展理论和实验工作。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


