Unraveling the nexus of oxidative stress, ocular diseases, and small extracellular vesicles to identify novel glaucoma biomarkers through in-depth proteomics
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
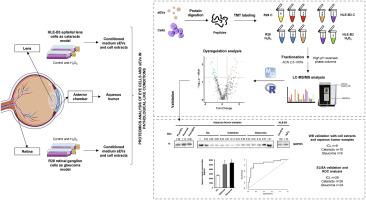
Chronic ocular pathologies such as cataracts and glaucoma are emerging as an important problem for public health due to the changes in lifestyle and longevity. These age-related ocular diseases are largely mediated by oxidative stress. Small extracellular vesicles (sEVs) are involved in cell-to-cell communication and transport. There is an increasing interest about the function of small extracellular vesicles (sEVs) in the eye. However, the proteome content and characterization of sEVs released by ocular cells under pathological conditions are not yet well known. Here, we aimed to analyze the protein profile of sEVs and the intracellular protein content from two ocular cell lines (lens epithelial cells and retinal ganglion cells) exposed to oxidative stress to identify altered proteins that could serve as potential diagnostic biomarkers. The protein content was analyzed by quantitative mass spectrometry-based proteomics. Validation was performed by WB and ELISA using cell extracts and aqueous humor from cataract and glaucoma patients. After data analysis, 176 and 7 dysregulated proteins with an expression ratio≥1.5 were identified in lens epithelial cells’ protein extract and sEVs, respectively, upon oxidative stress induction. In retinal ganglion cells, oxidative stress induction resulted in the dysregulation of 1033 proteins in cell extracts and 9 proteins in sEVs. In addition, by WB and ELISA, the dysregulation of proteins was mostly confirmed in aqueous humor samples from cataract or glaucoma patients in comparison to ICL individuals, with RAD23B showing high glaucoma diagnostic ability. Importantly, this work expands the knowledge of the proteome characterization of cataracts and glaucoma and provides new potential diagnostic glaucoma biomarkers.
通过深入的蛋白质组学研究揭示氧化应激、眼部疾病和细胞外小囊泡之间的联系,从而确定新型青光眼生物标志物
由于生活方式的改变和寿命的延长,白内障和青光眼等慢性眼部病变正在成为公共卫生的一个重要问题。这些与年龄有关的眼部疾病在很大程度上是由氧化应激介导的。细胞外小泡(sEVs)参与细胞间的交流和运输。人们对细胞外小泡(sEVs)在眼部的功能越来越感兴趣。然而,人们对眼部细胞在病理情况下释放的小细胞外囊泡的蛋白质组含量和特征还不甚了解。在这里,我们旨在分析暴露于氧化应激的两种眼细胞系(晶状体上皮细胞和视网膜神经节细胞)的 sEVs 蛋白概况和细胞内蛋白质含量,以确定可作为潜在诊断生物标志物的改变蛋白质。蛋白质含量通过基于质谱的定量蛋白质组学进行分析。利用白内障和青光眼患者的细胞提取物和房水,通过 WB 和 ELISA 进行了验证。经过数据分析,在氧化应激诱导下,晶状体上皮细胞蛋白提取物和sEVs中分别发现了176个和7个表达比例≥1.5的失调蛋白。在视网膜神经节细胞中,氧化应激诱导导致细胞提取物中 1033 个蛋白质和 sEV 中 9 个蛋白质表达失调。此外,通过WB和ELISA检测,白内障或青光眼患者的眼房水样本与ICL患者的眼房水样本相比,大部分都证实了蛋白质的失调,其中RAD23B显示出较高的青光眼诊断能力。重要的是,这项工作扩展了白内障和青光眼蛋白质组特征的知识,并提供了新的潜在青光眼诊断生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


