A potent Bioorganic azapodophyllotoxin derivative Suppresses tumor Progression in Triple negative breast Cancer: An Insight into its Inhibitory effect on tubulin polymerization and Disruptive effect on microtubule assembly
IF 5.4
3区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Triple negative breast cancer (TNBC) has long been a challenging disease owing to its high aggressive behaviour, poor prognosis and its limited treatment options. The growing demand of new therapeutics against TNBC enables us to examine the therapeutic efficiency of an emerging class of anticancer compounds, azapodophyllotoxin derivative (HTDQ), a nitrogen analogue of podophyllotoxin, using different biochemical, spectroscopic and computational approaches. The anticancer activities of HTDQ are studied by performing MTT assay in a dose depended manner on Triple negative breast cancer cells using MDA–MB-468 and MDA-MB-231 cell lines with IC50 value 937 nM and 1.13 µM respectively while demonstrating minimal effect on normal epithelial cells. The efficacy of HTDQ was further tested in 3D tumour spheroids formed by the human TNBC cell line MDA-MB468 and also the murine MMTV positive TNBC cell line 4 T1. The shrinkage that observed in the tumor spheroid clearly indicates that HTDQ remarkably decreases the growth of tumor spheroid thereby affirming its cytotoxicity. The 2D cell viability assay shows significant morphological alteration that possibly caused by the cytoskeleton disturbances. Hence the binding interaction of HTDQ with cytoskeleton protein tubulin, its effect on tubulin polymerisation as well as depolymerisation of preformed microtubules along with the conformational alternation in the protein itself have been investigated in detail. Moreover, the apoptotic effects of HTDQ have been examined using a range of apoptotic markers. HTDQ-treated cancer cells showed increased expression of cleaved PARP-1 and pro-caspase-3, suggesting activation of the apoptosis process. HTDQ also upregulated pro-apoptotic Bax expression while inhibiting anti-apoptotic Bcl2 expression, supporting its ability to induce apoptosis in cancer cells. Hence the consolidated biochemical and spectroscopic research described herein may provide enormous information to use azapodophyllotoxin as promising anticancer therapeutics for TNBC cells.
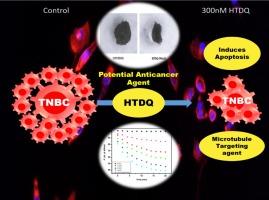
一种强效的无机氮杂鬼臼毒素衍生物可抑制三阴性乳腺癌的肿瘤进展:洞察其对微管蛋白聚合的抑制作用和对微管组装的破坏作用
长期以来,三阴性乳腺癌(TNBC)一直是一种具有挑战性的疾病,因为其侵袭性强、预后差且治疗方案有限。由于对 TNBC 新疗法的需求日益增长,我们采用不同的生化、光谱和计算方法,研究了一类新兴的抗癌化合物--氮杂鬼臼毒素衍生物 (HTDQ)(一种鬼臼毒素的氮类似物)的治疗效果。通过使用 MDA-MB-468 和 MDA-MB-231 细胞系对三阴性乳腺癌细胞进行 MTT 试验,研究了 HTDQ 的抗癌活性,其 IC50 值分别为 937 nM 和 1.13 µM,而对正常上皮细胞的影响极小。HTDQ 的功效还在由人类 TNBC 细胞系 MDA-MB468 和小鼠 MMTV 阳性 TNBC 细胞系 4 T1 形成的三维肿瘤球体内进行了进一步测试。在肿瘤球体内观察到的缩小现象清楚地表明,HTDQ 能显著减少肿瘤球体的生长,从而证实了其细胞毒性。二维细胞活力检测显示,细胞形态发生了显著变化,这可能是由细胞骨架紊乱引起的。因此,我们详细研究了 HTDQ 与细胞骨架蛋白微管蛋白的结合相互作用、其对微管蛋白聚合的影响、预形成微管的解聚以及微管蛋白本身的构象变化。此外,还使用一系列凋亡标记物研究了 HTDQ 的凋亡效应。经 HTDQ 处理的癌细胞中,已裂解的 PARP-1 和促 Caspase-3 的表达量增加,表明细胞凋亡过程被激活。HTDQ 还能上调促凋亡 Bax 的表达,同时抑制抗凋亡 Bcl2 的表达,支持其诱导癌细胞凋亡的能力。因此,本文所述的综合生化和光谱研究可为将氮杂鬼臼毒素用作治疗TNBC细胞的抗癌药物提供大量信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Energy Materials
Materials Science-Materials Chemistry
CiteScore
10.30
自引率
6.20%
发文量
1368
期刊介绍:
ACS Applied Energy Materials is an interdisciplinary journal publishing original research covering all aspects of materials, engineering, chemistry, physics and biology relevant to energy conversion and storage. The journal is devoted to reports of new and original experimental and theoretical research of an applied nature that integrate knowledge in the areas of materials, engineering, physics, bioscience, and chemistry into important energy applications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


