Carvacrol-conjugated 3-Hydroxybenzoic Acids: Design, Synthesis, cardioprotective potential against doxorubicin-induced Cardiotoxicity, and ADMET study
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Carvacrol (CA) is a phenolic monoterpene renowned for its diverse pharmacological benefits, particularly its cardioprotective effects. Concurrently, phenolic acids have also demonstrated promise in mitigating drug-induced cardiotoxicity. Focusing on combating doxorubicin-induced cardiotoxicity (DIC), the research aims to synthesize novel cardioprotective agents by combining CA with 3-hydroxybenzoic acid (3HA). Doxorubicin, an anticancer drug, poses cardiovascular risks as its adverse effect, prompting the exploration of hybrid compounds. Various linker molecules, including alkyl and acyl with different carbon lengths, were investigated to understand their impact on bioactivity. In vitro testing on the DOX-induced H9c2 cell death model revealed the effectiveness of a CA conjugate in preserving cardiomyocyte viability. In silico analysis highlighted favorable drug-like properties and low toxicity of the conjugate. This study sheds light on molecular hybridization’s potential in developing cardioprotective agents, emphasizing CA’s pivotal role in combating DIC.
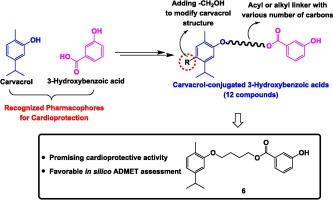
香芹酚共轭 3-羟基苯甲酸:设计、合成、针对多柔比星诱导的心脏毒性的心脏保护潜力以及 ADMET 研究
香芹酚(CA)是一种酚类单萜,因其多种药理作用而闻名,尤其是对心脏的保护作用。同时,酚酸在减轻药物引起的心脏毒性方面也表现出了良好的前景。这项研究以对抗多柔比星诱导的心脏毒性(DIC)为重点,旨在通过将 CA 与 3-hydroxybenzoic acid(3HA)结合,合成新型心脏保护剂。多柔比星是一种抗癌药物,其不良反应对心血管造成危害,这促使人们探索混合化合物。我们研究了各种连接分子,包括不同碳长的烷基和酰基,以了解它们对生物活性的影响。对 DOX 诱导的 H9c2 细胞死亡模型进行的体外测试表明,CA 结合物能有效保持心肌细胞的活力。硅学分析强调了该共轭物具有良好的类药物特性和低毒性。这项研究揭示了分子杂交在开发心脏保护剂方面的潜力,强调了 CA 在抗 DIC 中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


