Hypergolic fuel impacting a gelled oxidizer wall: Droplet dynamics, heat release, ignition, and flame analysis
IF 2.8
2区 工程技术
Q2 ENGINEERING, MECHANICAL
Experimental Thermal and Fluid Science
Pub Date : 2024-09-24
DOI:10.1016/j.expthermflusci.2024.111322
引用次数: 0
Abstract
The combination of high-concentration solutions of hydrogen peroxide, known as High Test Peroxide (HTP), with the green fuel composed of tetramethylethylenediamine, dimethylaminoethanol, and methanol (TMEDA/DMEA/MeOH, 1:1:1 vol%), catalyzed by 1 wt% of copper chloride dihydrate, has significant potential for space hypergolic propulsion applications in terms of performance and safe operation. Besides the well-known and mature propulsion systems adopting liquid and solid propellants, gel propellants also present interesting characteristics that can be better explored to enable the creation of alternative propulsive systems. The present work employed HTP at 98 wt% with 6 wt% of fumed silica as a gelling agent to create gelled HTP (GHTP). Droplets of the green fuel impinged on a layer of GHTP placed over a glass slide, acting as a solid oxidizer, mimicked an element of a new hybrid gel/liquid hypergolic propulsion system. Data from infrared and visible light cameras enabled a detailed analysis of the dynamics of a reactive droplet impinging on a gelled oxidizer wall, as well as the heat release, ignition, and flame spread of the hypergolic GHTP/green fuel pair under open-air conditions. The heat release was observed to be distributed across concentric annular areas for both the fuel surrogate and the fuel with catalyst, before and after ignition, demonstrating a strong correlation with non-reactive droplet fluid dynamics, which was corroborated by spread diameter analysis processed from visible image data. Vapor and Ignition Delay Times (VDT and IDT) were found to be as low as 4 and 17 ms, respectively, for the highest droplet impact velocity and catalyst concentration. The reaction rate in the gas phase, considering the flame spread area, showed a dependency of both impact velocity and catalyst concentration, with the latter exhibiting a more pronounced and clear effect. The surface temperature ranges where first vaporization and ignition occurred were 60 °C 70 °C and 120 170 °C, respectively, which is close to the boiling point of methanol and the auto-ignition temperature of TMEDA. This finding, along with the chemical mechanisms in the gas phase related to the presence of a catalyst in the fuel, may be important for hypergolic ignition. The relatively low ignition temperatures and the short ignition times represent additional advantages of the present hypergolic combination for propulsion applications.
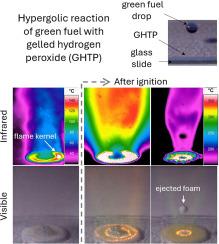
过热燃料撞击凝胶氧化剂壁:液滴动力学、热释放、点火和火焰分析
高浓度过氧化氢溶液(称为高试验过氧化氢(HTP))与由四甲基乙二胺、二甲基氨基乙醇和甲醇(TMEDA/DMEA/MeOH,1:1:1 vol%)组成的绿色燃料(1 wt%的二水氯化铜催化)的结合,在性能和安全操作方面具有空间超高压推进应用的巨大潜力。除了采用液体和固体推进剂的众所周知的成熟推进系统外,凝胶推进剂也呈现出一些有趣的特性,可以更好地加以探索,以创建替代推进系统。本研究采用含 98 wt% HTP 和 6 wt% 气相二氧化硅作为胶凝剂,制造出胶凝 HTP(GHTP)。绿色燃料滴落在玻璃载玻片上的 GHTP 层上,充当固体氧化剂,模拟了新型凝胶/液体混合双醇推进系统的一个要素。通过红外线和可见光摄像机获得的数据,可以详细分析反应液滴撞击胶状氧化剂壁的动态,以及双酚 GHTP/绿色燃料在露天条件下的热释放、点火和火焰蔓延情况。在点火前和点火后,观察到代用燃料和带催化剂燃料的热量释放都分布在同心环形区域,这表明与非反应液滴流体动力学密切相关,根据可见光图像数据处理的扩散直径分析也证实了这一点。在液滴冲击速度和催化剂浓度最高的情况下,蒸发和点火延迟时间(VDT 和 IDT)分别低至 4 毫秒和 17 毫秒。考虑到火焰扩散面积,气相中的反应速率与撞击速度和催化剂浓度有关,后者的影响更为明显和清晰。首次汽化和着火的表面温度范围分别为 60 °C ~ 70 °C 和 120 ~ 170 °C,接近甲醇的沸点和 TMEDA 的自燃温度。这一发现以及气相中与燃料中催化剂的存在有关的化学机制可能对双酚点火非常重要。相对较低的点火温度和较短的点火时间是本超醇类组合在推进应用方面的额外优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Thermal and Fluid Science
工程技术-工程:机械
CiteScore
6.70
自引率
3.10%
发文量
159
审稿时长
34 days
期刊介绍:
Experimental Thermal and Fluid Science provides a forum for research emphasizing experimental work that enhances fundamental understanding of heat transfer, thermodynamics, and fluid mechanics. In addition to the principal areas of research, the journal covers research results in related fields, including combined heat and mass transfer, flows with phase transition, micro- and nano-scale systems, multiphase flow, combustion, radiative transfer, porous media, cryogenics, turbulence, and novel experimental techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


