Facile CH aryl and OH bonds activation at dirhenium site of [Re2(CO)8(THF)2] complex
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
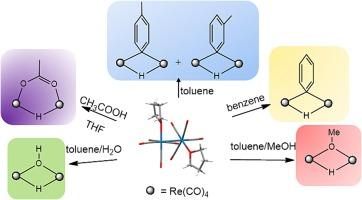
The activation of small molecules is a topic of great interest and previously we reported on the easy activation of different types of E![]() H bonds at the dinuclear complex [Re2(CO)8(THF)2] (1) where labile THF molecules coordinate to adjacent rhenium(0) atoms. Here we extend the reactivity of 1 reporting on the oxidative addition of benzene and toluene at room temperature to give [Re2(μ-H)(μ-κC-Ar)(CO)8], Ar = −C6H5 (2) and −C6H4Me (3). Compound 3 is a new example of μ-κC-Ar dinuclear rhenium complex and has been obtained as para and meta isomers (3a,b). It is known from the literature that 2 can activate arenes and heteroarenes via reductive elimination of benzene and oxidative addition of C
H bonds at the dinuclear complex [Re2(CO)8(THF)2] (1) where labile THF molecules coordinate to adjacent rhenium(0) atoms. Here we extend the reactivity of 1 reporting on the oxidative addition of benzene and toluene at room temperature to give [Re2(μ-H)(μ-κC-Ar)(CO)8], Ar = −C6H5 (2) and −C6H4Me (3). Compound 3 is a new example of μ-κC-Ar dinuclear rhenium complex and has been obtained as para and meta isomers (3a,b). It is known from the literature that 2 can activate arenes and heteroarenes via reductive elimination of benzene and oxidative addition of C![]() H bonds to the dinuclear fragment. Here we have studied the reaction of 2 with C6D6 and H2C
H bonds to the dinuclear fragment. Here we have studied the reaction of 2 with C6D6 and H2C![]() C(H)Ph and determined the kinetic constants by 1H NMR (1.4 × 10−5 s−1 at 308 K and 1.1 × 10−5 s−1 at 298 K, respectively). The results indicate that the rate-determining step of the reaction is the reductive elimination of benzene, while the oxidative addition is fast. Water and methanol react with 1 in toluene at room temperature to give the hydroxo and methoxo hydrido complexes [Re2(μ-H)(μ-OR)(CO)8], R
C(H)Ph and determined the kinetic constants by 1H NMR (1.4 × 10−5 s−1 at 308 K and 1.1 × 10−5 s−1 at 298 K, respectively). The results indicate that the rate-determining step of the reaction is the reductive elimination of benzene, while the oxidative addition is fast. Water and methanol react with 1 in toluene at room temperature to give the hydroxo and methoxo hydrido complexes [Re2(μ-H)(μ-OR)(CO)8], R![]() H (5) and CH3 (6). On reacting 1 with water in deuterated toluene, and monitoring by 1H/2H NMR, a preferential deuteration of the hydride site to give [Re2(μ-D)(μ-OH)(CO)8] is evidenced. This finding excludes the oxidative addition of water on the dinuclear “Re2(CO)8” fragment while supporting a heterolytic addition of water via protonation at the µ-κC-tolyl group, elimination of toluene and addition of OH−. Single crystal X-ray diffraction analyses have been performed for complexes 3a, 5 and 6 and their solid state structures have been determined. In particular, the crystal structure of 5 results in a new polymorphic form (5b) and it is discussed in comparison with the already known one (5a).
H (5) and CH3 (6). On reacting 1 with water in deuterated toluene, and monitoring by 1H/2H NMR, a preferential deuteration of the hydride site to give [Re2(μ-D)(μ-OH)(CO)8] is evidenced. This finding excludes the oxidative addition of water on the dinuclear “Re2(CO)8” fragment while supporting a heterolytic addition of water via protonation at the µ-κC-tolyl group, elimination of toluene and addition of OH−. Single crystal X-ray diffraction analyses have been performed for complexes 3a, 5 and 6 and their solid state structures have been determined. In particular, the crystal structure of 5 results in a new polymorphic form (5b) and it is discussed in comparison with the already known one (5a).
在[Re2(CO)8(THF)2]络合物的钌位点轻松激活 CH 芳基键和 OH 键
小分子的活化是一个备受关注的话题,之前我们曾报道过双核复合物 [Re2(CO)8(THF)2] (1) 易于活化不同类型的 EH 键,其中易变的 THF 分子与相邻的铼(0)原子配位。在此,我们扩展了 1 的反应性,报告了苯和甲苯在室温下的氧化加成反应,得到了 [Re2(μ-H)(μ-κC-Ar)(CO)8],Ar = -C6H5 (2) 和 -C6H4Me (3)。化合物 3 是 μ-κC-Ar 双核铼络合物的一个新例子,以对位和元异构体形式出现(3a,b)。从文献中得知,2 可以通过苯的还原消除和双核片段 CH 键的氧化加成来活化炔和杂环烯。在此,我们研究了 2 与 C6D6 和 H2CC(H)Ph 的反应,并通过 1H NMR 测定了动力学常数(308 K 时分别为 1.4 × 10-5 s-1 和 298 K 时分别为 1.1 × 10-5 s-1)。结果表明,决定反应速率的步骤是苯的还原消除,而氧化加成的速度很快。水和甲醇在室温下与 1 在甲苯中反应,生成氢氧基和甲氧基氢化物[Re2(μ-H)(μ-OR)(CO)8]、RH (5) 和 CH3 (6)。将 1 与水在氚代甲苯中反应,并通过 1H/2H NMR 进行监测,结果表明氢化物位点优先发生氚化反应,生成 [Re2(μ-D)(μ-OH)(CO)8]。这一发现排除了水在双核 "Re2(CO)8 "片段上的氧化加成,同时支持水通过µ-κC-甲苯基团的质子化、甲苯的消除和 OH- 的加成进行异溶解加成。对复合物 3a、5 和 6 进行了单晶 X 射线衍射分析,并确定了它们的固态结构。特别是 5 的晶体结构产生了一种新的多晶型(5b),并与已知的多晶型(5a)进行了比较讨论。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


