LocoMMotion: a study of real-life current standards of care in triple-class exposed patients with relapsed/refractory multiple myeloma – 2-year follow-up (final analysis)
IF 12.8
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
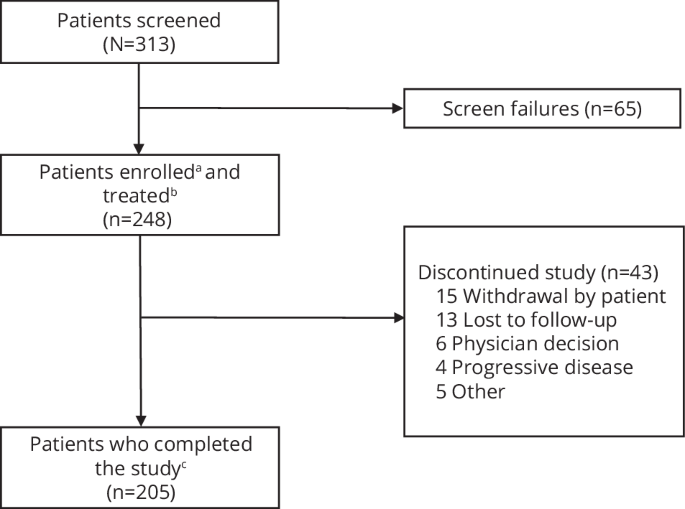
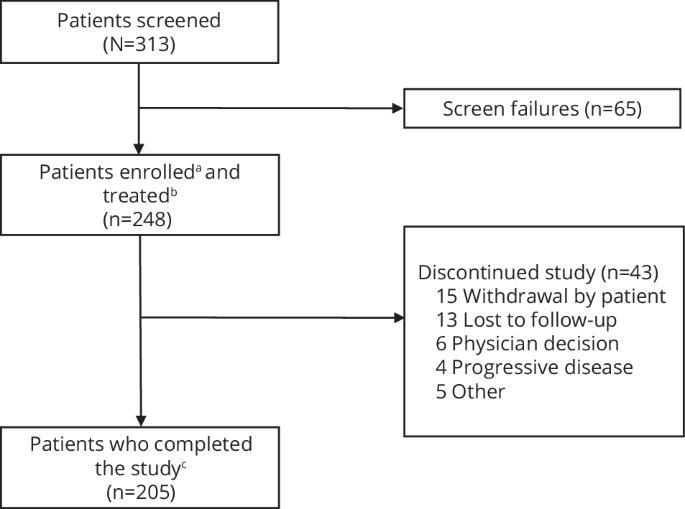
Treatment of relapsed/refractory multiple myeloma (RRMM) is challenging as patients exhaust all available therapies and the disease becomes refractory to standard drug classes. Here we report the final results of LocoMMotion, the first prospective study of real-world clinical practice (RWCP) in triple-class exposed (TCE) patients with RRMM, with a median follow-up of 26.4 months (range, 0.1–35.0). Patients (N = 248) had received median 4 prior LOT (range, 2–13) at enrollment. 91 unique regimens were used in index LOT. Overall response rate was 31.9% (95% CI, 26.1–38.0), median progression-free survival (PFS) was 4.6 months (95% CI, 3.9–5.6) and median overall survival was 13.8 months (95% CI, 10.8–17.0). 152 patients (61.3%) had subsequent LOTs with 134 unique regimens, of which 78 were used in first subsequent LOT. Median PFS2 (from start of study through first subsequent LOT) was 10.8 months (95% CI, 8.4–13.0). 158 patients died on study, 67.7% due to progressive disease. Additional subgroup analyses and long-term safety summaries are reported. The high number of RWCP treatment regimens utilized and poor clinical outcomes confirm a lack of standardized treatment for TCE patients with RRMM, highlighting the need for new treatments with novel mechanisms.
LocoMMotion:针对复发性/难治性多发性骨髓瘤三类暴露患者的现实生活中现行护理标准研究--2 年随访(最终分析)
复发性/难治性多发性骨髓瘤(RRMM)的治疗具有挑战性,因为患者用尽了所有可用的疗法,而且疾病对标准药物类别产生了耐药性。在此,我们报告了LocoMMotion的最终结果,这是首个针对暴露于三类药物(TCE)的RRMM患者进行的真实世界临床实践(RWCP)前瞻性研究,中位随访时间为26.4个月(0.1-35.0个月)。患者(N = 248)入组时中位接受过 4 次 LOT 治疗(2-13 次)。指数 LOT 采用了 91 种不同的治疗方案。总反应率为31.9%(95% CI,26.1-38.0),中位无进展生存期(PFS)为4.6个月(95% CI,3.9-5.6),中位总生存期为13.8个月(95% CI,10.8-17.0)。152名患者(61.3%)接受了后续LOT治疗,使用了134种独特的治疗方案,其中78种用于首次后续LOT治疗。中位 PFS2(从研究开始到首次后续治疗)为 10.8 个月(95% CI,8.4-13.0)。158名患者在研究期间死亡,67.7%死于疾病进展。本研究还报告了其他亚组分析和长期安全性总结。使用的RWCP治疗方案较多,临床疗效不佳,这证实了TCE RRMM患者缺乏标准化治疗,突出了对具有新机制的新疗法的需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


