Biophysically interpretable inference of cell types from multimodal sequencing data
IF 12
Q1 COMPUTER SCIENCE, INTERDISCIPLINARY APPLICATIONS
引用次数: 0
Abstract
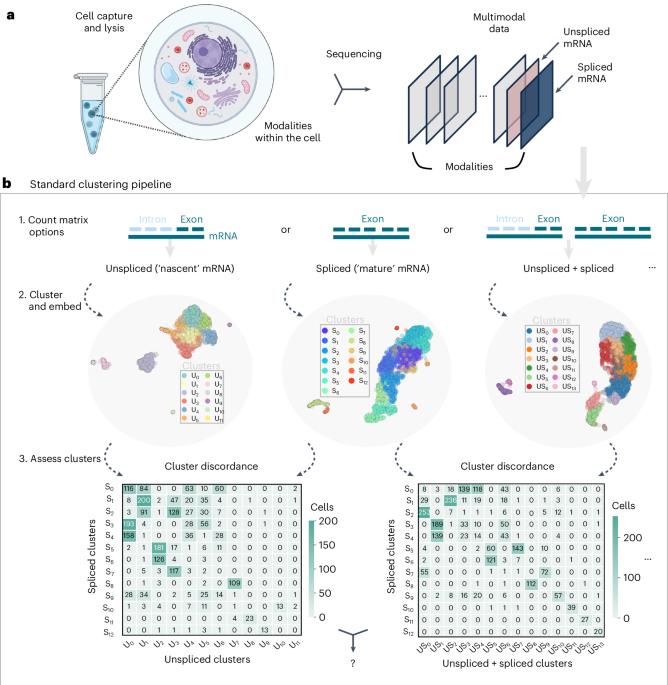
Multimodal, single-cell genomics technologies enable simultaneous measurement of multiple facets of DNA and RNA processing in the cell. This creates opportunities for transcriptome-wide, mechanistic studies of cellular processing in heterogeneous cell populations, such as regulation of cell fate by transcriptional stochasticity or tumor proliferation through aberrant splicing dynamics. However, current methods for determining cell types or ‘clusters’ in multimodal data often rely on ad hoc approaches to balance or integrate measurements, and assumptions ignoring inherent properties of the data. To enable interpretable and consistent cell cluster determination, we present meK-means (mechanistic K-means) which integrates modalities through a unifying model of transcription to learn underlying, shared biophysical states. With meK-means we can cluster cells with nascent and mature mRNA measurements, utilizing the causal, physical relationships between these modalities. This identifies shared transcription dynamics across cells, which induce the observed molecule counts, and provides an alternative definition for ‘clusters’ through the governing parameters of cellular processes. MeK-means clusters single-cell multimodal data by linking modalities through their biophysical relationships. We redefine clusters through transcription kinetics to reveal how RNA production and processing drive cellular diversity and disease.
从多模态测序数据推断细胞类型的生物物理可解释性
多模态单细胞基因组学技术可同时测量细胞中 DNA 和 RNA 处理的多个方面。这为对异质细胞群中的细胞处理过程进行全转录组机理研究创造了机会,例如通过转录随机性调节细胞命运或通过异常剪接动态调节肿瘤增殖。然而,目前在多模态数据中确定细胞类型或 "集群 "的方法往往依赖于平衡或整合测量数据的特别方法,以及忽略数据固有特性的假设。为了实现可解释且一致的细胞群确定,我们提出了 meK-means(机械 K-means),它通过统一的转录模型整合各种模式,以了解潜在的、共享的生物物理状态。有了 meK-means,我们就能利用这些模式之间的因果物理关系,通过对新生和成熟 mRNA 的测量对细胞进行聚类。这就确定了细胞间共享的转录动态,从而诱导观察到的分子数量,并通过细胞过程的管理参数为 "群集 "提供了另一种定义。MeK-means 通过生物物理关系将各种模式联系起来,从而对单细胞多模态数据进行聚类。我们通过转录动力学重新定义集群,揭示 RNA 的产生和处理如何驱动细胞多样性和疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


