Potential siderophore-dependent mutualism in the harmful dinoflagellate Alexandrium pacificum (Group IV) and bacterium Photobacterium sp. TY1-4 under iron-limited conditions
IF 5.5
1区 生物学
Q1 MARINE & FRESHWATER BIOLOGY
引用次数: 0
Abstract
Specific bacterial species induce algal blooms by producing growth-promoting substances, such as siderophores, under iron-limited conditions. However, the molecular mechanisms underlying these effects remain poorly understood. This study investigates the interactions between the harmful dinoflagellate Alexandrium pacificum (Group IV) and siderophore-producing bacteria, with a focus on iron acquisition facilitated by bacterial siderophores. During algal bloom seasons in the South Sea of Korea, Photobacterium sp. TY1-4 was isolated, which enhances A. pacificum cell density under iron-deficient conditions, TY1-4 can use the sterile exudates from A. pacificum as the sole source of carbon, suggesting a mutualistic relationship. Transcriptomic and genomic analyses revealed siderophore-mediated redox-based signaling and non-reductive pathways enhancing iron bioavailability. Photobacterium sp. TY1-4 initiates siderophore production through quorum sensing, whereas A. pacificum utilizes specific receptors and transporters for hydroxamate-type siderophores (ApFHUA and ApFHUC) to uptake iron. Three redox key iron-uptake genes were also identified in A. pacificum: membrane-bound ferroxidase ApFET3, high-affinity iron permease ApFTR1, and ferric-chelate reductases/oxidoreductases ApFRE1, with transcription levels inversely related to bioavailable iron. Increased iron bioavailability mediated by siderophores alleviates iron stress in A. pacificum, supporting its growth in iron-scarce environments. Additionally, A. pacificum co-cultured with Photobacterium sp. TY1-4 synthesized high-toxicity STXs, including GTX4, GTX2, and STX. These findings highlight the critical role of bacterial siderophores in iron binding and their potential impact on harmful algal bloom dynamics.
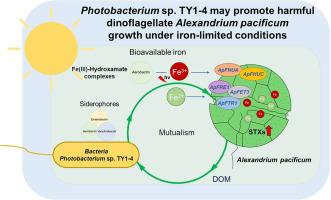
有害甲藻亚历山大藻(第 IV 组)与光杆菌 TY1-4 在铁限制条件下可能存在的依赖苷元的互生关系
在铁有限的条件下,特定的细菌物种会产生促进生长的物质,如嗜苷酸盐,从而诱发藻华。然而,人们对这些效应的分子机制仍然知之甚少。本研究调查了有害甲藻亚历山大藻(第 IV 组)与产生嗜凫藻的细菌之间的相互作用,重点是细菌嗜凫藻促进铁的获取。在韩国南海藻类大量繁殖的季节,分离出了光杆菌 TY1-4,它能在缺铁条件下提高太平洋亚历山大藻的细胞密度,TY1-4 能利用太平洋亚历山大藻的无菌渗出物作为唯一的碳源,这表明两者之间存在互利关系。转录组和基因组分析揭示了苷元介导的基于氧化还原的信号传导和非还原途径,可提高铁的生物利用率。光杆菌(Photobacterium sp. TY1-4)通过法定人数感应(quorum sensing)产生苷酸,而太平洋蝇(A. pacificum)则利用羟基氨基甲酸酯型苷酸的特异受体和转运体(ApFHUA 和 ApFHUC)来吸收铁。在太平洋蛙中还发现了三个氧化还原关键铁吸收基因:膜结合铁氧化酶 ApFET3、高亲和力铁渗透酶 ApFTR1 和铁螯合还原酶/氧化还原酶 ApFRE1,其转录水平与生物可用铁成反比。嗜苷铁元素介导的铁生物利用率的增加缓解了太平洋蛙的铁压力,支持其在缺铁环境中生长。此外,与光杆菌 TY1-4 共同培养的太平洋蛙能合成高毒性 STX,包括 GTX4、GTX2 和 STX。这些发现凸显了细菌嗜苷酸盐在铁结合中的关键作用及其对有害藻华动态的潜在影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Harmful Algae
生物-海洋与淡水生物学
CiteScore
12.50
自引率
15.20%
发文量
122
审稿时长
7.5 months
期刊介绍:
This journal provides a forum to promote knowledge of harmful microalgae and macroalgae, including cyanobacteria, as well as monitoring, management and control of these organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


