Dual S-scheme Bi2MoO6/g-C3N4/Ag2MoO4 ternary heterojunction: Interfacial charge transfer, broadband spectrum, enhanced redox ability
IF 3.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
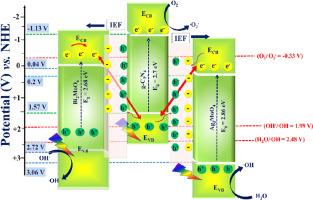
A ternary heterojunction bearing Bi2MoO6 and g-C3N4 is deposited on Ag2MoO4 for the photocatalytic degradation of sulfamethoxazole (SMX) antibiotic. Though the hydrothermal synthesis is non-directional, the dual S- scheme heterojunction formation is governed by the g-C3N4 serving as an electron bridge between Bi2MoO6 and Ag2MoO4. The potent strong interaction with both Bi2MoO6 and Ag2MoO4 facilitates high oxidation and reduction potential. The optimized Bi2MoO6/g-C3N4/Ag2MoO4 heterojunction with extended visible light absorption exhibits 96 % SMX degradation efficiency within 240 min of irradiations. The dual S-scheme configuration endows in-built electric field with vigorous driving force for charge carrier separation. The charge transfer mechanisms were validated by the photoluminescence results. Bi2MoO6/g-C3N4/Ag2MoO4 heterojunction demonstrates pseudo-first order kinetics with 0.143 kmin−1 for SMX degradation and ternary photocatalyst 83 % degraded SMX after successive five cycles. In the formed dual S-scheme Bi2MoO6/g-C3N4/Ag2MoO4heterojunction, ●OH and ●O2− radicals were the main reactive species for SMX degradation. This research contributes to the formation of stable multicomponent photocatalytic systems.
双 S 型 Bi2MoO6/g-C3N4/Ag2MoO4 三元异质结:界面电荷转移、宽带光谱、增强的氧化还原能力
在 Ag2MoO4 上沉积了含 Bi2MoO6 和 g-C3N4 的三元异质结,用于光催化降解磺胺甲噁唑(SMX)抗生素。虽然水热合成是非定向的,但双 S 方案异质结的形成是由 g-C3N4 作为 Bi2MoO6 和 Ag2MoO4 之间的电子桥所决定的。g-C3N4 与 Bi2MoO6 和 Ag2MoO4 之间的强相互作用促进了高氧化和还原电位的形成。经过优化的 Bi2MoO6/g-C3N4/Ag2MoO4 异质结具有扩展的可见光吸收能力,在 240 分钟的照射时间内,SMX 降解效率达到 96%。双 S 型结构为电荷载流子分离提供了强大的内置电场驱动力。光致发光结果验证了电荷转移机制。Bi2MoO6/g-C3N4/Ag2MoO4 异质结以 0.143 kmin-1 的假一阶动力学降解了 SMX,三元光催化剂在连续五个周期后 83% 降解了 SMX。在所形成的双 S 型 Bi2MoO6/g-C3N4/Ag2MoO4 异质结中,●OH 和 ●O2- 自由基是降解 SMX 的主要反应物。这项研究有助于形成稳定的多组分光催化系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


