Mononuclear high-spin iron(III) phthalocyanines
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
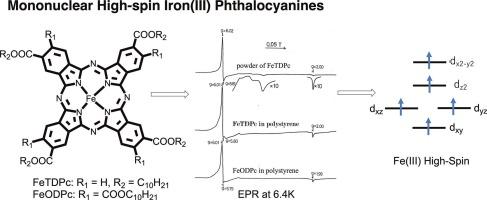
2,9(or 10),16(or 17), 23(or 24)-Tetradecyloxycarbonylphthalocyaninatoiron, FeTDPc, and 2,3,9,10,16,17,23,24-octadecyloxycarbonylphthalocyaninatoiron, FeODPc, were synthesized and characterized. These compounds seem to be in trivalent iron high-spin state in solvents such as chloroform, dichloromethane, benzene, and chlorobenzene, although their counter anion could not be detected by elemental analyses. They react with strong bases such as pyridine and imidazoles to form their mono- and subsequently their di-base complexes with formation constant of >106 and < 200 dm3 mol−1, respectively, in dichloromethane at 20 °C. The resultant mono-adducts appear to be trivalent iron low-spin while the di-base adducts are bivalent iron low-spin state complexes. The addition of ca. 10–30 equivalent of tetrabutylammonium-chloride or -bromide (electrolyte) to the solution containing FeTDPc or FeODPc, causes their spin-state change from iron(III) high to low-spin state. In a solid power state, however, both FeTDPc and FeODPc exist as a mixture of high-spin iron(III)- and intermediate-spin iron(II) species. Strangely, when these compounds are dissolved in polystyrene, i.e. each molecules are isolated from each other, the signals originated from the iron(II) component disappear.
单核高自旋铁(III)酞菁
合成并鉴定了 2,9(或 10),16(或 17),23(或 24)-十四烷氧基羰基酞菁铁(FeTDPc)和 2,3,9,10,16,17,23,24-十八烷氧基羰基酞菁铁(FeODPc)。这些化合物在氯仿、二氯甲烷、苯和氯苯等溶剂中似乎处于三价铁高自旋态,但其反阴离子无法通过元素分析检测到。在 20 °C 的二氯甲烷中,它们会与吡啶和咪唑等强碱发生反应,形成单碱和双碱络合物,形成常数分别为 106 和 200 dm3 mol-1。生成的单加合物似乎是三价低旋铁,而二碱加合物则是二价低旋铁态络合物。在含有 FeTDPc 或 FeODPc 的溶液中加入约 10-30 等量的四丁基氯化铵或溴化铵(电解质),会使它们的自旋态从铁(III)的高自旋态转变为低自旋态。然而,在固态电源状态下,FeTDPc 和 FeODPc 都是高自旋铁(III)和中等自旋铁(II)的混合物。奇怪的是,当这些化合物溶解在聚苯乙烯中时,即各分子相互隔离时,来自铁(II)成分的信号消失了。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


