Effect of backbone linker (O vs NH) on the ability of pincer-supported nickel hydrides to reduce CO2
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The reactivity of nickel hydrides supported by a pincer ligand often can be tuned by modifying the ligand at the three donor sites. In this study, ligand modification is made at the backbone, more specifically the linkers X and Y in {2,6-C6H3(XPtBu2)(YPtBu2)}NiH. Of the three nickel hydrides investigated herein, the PNCNP-pincer complex (X = Y = NH) is the most reactive one towards CO2, whether it is for the rate of CO2 capture from air or for the thermodynamic favorability of CO2 insertion into the Ni–H bond. The POCOP-pincer complex (X = Y = O) is the least reactive hydride whereas the POCNP-pincer complex (X = O, Y = NH) is ranked in the middle. To have access to the hybrid pincer complex, an improved synthetic method for the proligand, m-C6H4(OPtBu2)(NHPtBu2), is also developed.
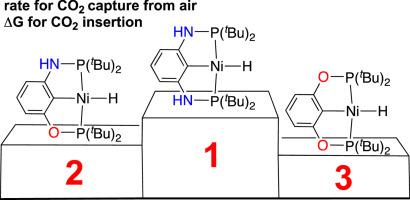
骨架连接物(O 与 NH)对钳撑镍氢化物还原 CO2 能力的影响
钳形配体支持的镍氢化物的反应活性通常可以通过在三个供体位点上对配体进行修饰来调整。在本研究中,配体的修饰是在骨架上进行的,更具体地说是在{2,6-C6H3(XPtBu2)(YPtBu2)}NiH中的连接体X和Y上进行的。在本文研究的三种镍氢化物中,PNCNP-pincer 复合物(X = Y = NH)对二氧化碳的反应性最强,无论是从空气中捕获二氧化碳的速率,还是从二氧化碳插入镍-H 键的热力学有利条件来看,都是如此。POCOP-pincer 复合物(X = Y = O)是反应性最小的氢化物,而 POCNP-pincer 复合物(X = O,Y = NH)则处于中间位置。为了获得混合钳状配合物,还开发了一种改进的原配体 m-C6H4(OPtBu2)(NHPtBu2)合成方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


