Achieving over 90% Faradaic Efficiency in Cyclohexanone Oxime Electrosynthesis Using the Cu–Mo Dual-Site Catalyst
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Coupling with the nitrate electroreduction reaction (NitRR), the electrosynthesis of cyclohexanone oxime (CHO, the vital feedstock in the nylon-6 industry) from cyclohexanone provides a promising alternative to the traditional energy consumption process. However, it still suffers from low efficiency because selective production of *NH2OH intermediate from NitRR under large current densities is challenging. We here report a Cu1MoOx/nitrogen-doped carbon (NC) electrocatalyst with high-density Cu–Mo dual sites for NitRR to selectively produce and stabilize *NH2OH, with the subsequent cyclohexanone oximation achieving the highest CHO Faradaic efficiency of 94.5% and a yield rate of 3.0 mol g–1 h–1 at an industrially relevant current density of 0.5 A cm–2. Furthermore, in situ characterizations evidenced that the Cu–Mo dual sites in Cu1MoOx/NC effectively inhibited hydrodeoxygenation of hydroxyl-containing intermediates of NitRR, selectively producing *NH2OH and thus achieving cyclohexanone oximation with high efficiency. This work provides a high-performance catalyst for CHO electrosynthesis from nitrogenous waste, showing promising application potential in industrial production of CHO.
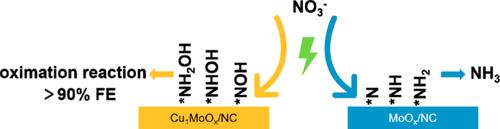
使用铜-钼双位催化剂在环己酮肟电合成中实现 90% 以上的法拉第效率
与硝酸盐电还原反应(NitRR)相结合,从环己酮电合成环己酮肟(CHO,尼龙-6 工业的重要原料)为传统的能耗工艺提供了一种前景广阔的替代方法。然而,由于在大电流密度下从 NitRR 中选择性地生产 *NH2OH 中间体具有挑战性,因此该工艺仍然存在效率低的问题。我们在此报告了一种 Cu1MoOx/掺氮碳(NC)电催化剂,该催化剂具有高密度的 Cu-Mo 双位点,可用于 NitRR 选择性地产生并稳定 *NH2OH,随后的环己酮氧化在工业相关的 0.5 A cm-2 电流密度下实现了 94.5% 的最高 CHO 法拉第效率和 3.0 mol g-1 h-1 的产率。此外,原位表征证明,Cu1MoOx/NC 中的 Cu-Mo 双位点能有效抑制 NitRR 含羟基中间体的加氢脱氧反应,选择性地产生 *NH2OH,从而高效地实现环己酮氧化。这项研究为利用含氮废物电合成 CHO 提供了一种高性能催化剂,在 CHO 的工业生产中具有广阔的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


