The Photoredox Paradox: Electron and Hole Upconversion as the Hidden Secrets of Photoredox Catalysis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Although photoredox catalysis is complex from a mechanistic point of view, it is also often surprisingly efficient. In fact, the quantum efficiency of a puzzlingly large portion of photoredox reactions exceeds 100% (i.e., the measured quantum yields (QYs) are >1). Hence, these photoredox reactions can be more than perfect with respect to photon utilization. In several documented cases, a single absorbed photon can lead to the formation of >100 molecules of the product, behavior known to originate from chain processes. In this Perspective, we explore the underlying reasons for this efficiency, identify the nature of common catalytic chains, and highlight the differences between HAT and SET chains. Our goal is to show why chains are especially important in photoredox catalysis and where the thermodynamic driving force that sustains the SET catalytic cycles comes from. We demonstrate how the interplay of polar and radical processes can activate hidden catalytic pathways mediated by electron and hole transfer (i.e., electron and hole catalysis). Furthermore, we illustrate how the phenomenon of redox upconversion serves as a thermodynamic precondition for electron and hole catalysis. After discussing representative mechanistic puzzles, we analyze the most common bond forming steps, where redox upconversion frequently occurs (and issometimes unavoidable). In particular, we highlight the importance of 2-center-3-electron bonds as a recurring motif that allows a rational chemical approach to the design of redox upconversion processes.
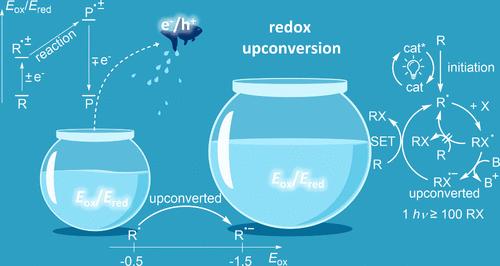
光氧化悖论:电子和空穴上转换是光氧化催化的秘密
尽管从机理的角度来看,光氧化催化反应非常复杂,但其效率却往往出人意料。事实上,有很大一部分光氧化还原反应的量子效率超过了 100%(即测得的量子产率(QYs)为 1),令人费解。因此,在光子利用率方面,这些光氧化反应可以说是完美无缺的。在一些记录在案的案例中,一个被吸收的光子可导致形成 100 个分子的产物,这种行为已知源于链式反应过程。在本《视角》中,我们将探讨这种效率的根本原因,确定常见催化链的性质,并强调 HAT 链和 SET 链之间的差异。我们的目标是说明为什么链在光氧化催化中尤为重要,以及维持 SET 催化循环的热动力来自何处。我们展示了极性和自由基过程的相互作用如何激活由电子和空穴传输(即电子和空穴催化)介导的隐藏催化途径。此外,我们还说明了氧化还原上转换现象如何成为电子和空穴催化的热力学先决条件。在讨论了具有代表性的机理难题之后,我们分析了最常见的成键步骤,在这些步骤中经常会发生氧化还原上转换现象(有时甚至是不可避免的)。我们特别强调了 2-中心-3-电子键作为一种重复出现的主题的重要性,它允许采用合理的化学方法来设计氧化还原上转换过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


