Oxoanion complexation of nitroisophthalamide receptors: Insights from the DFT calculations
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
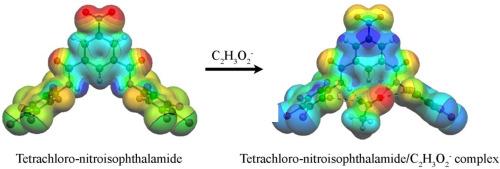
Amide derivative receptors have been designed to investigate the oxoanion complexation ability via hydrogen and halogen bond interactions. Structural, energetic and electronic properties of nitroisophthalamide receptors, i.e., di(benzyl)− (R1), di(hexafluoro)− (R2), di(chloro−,tetrafluoro)− (R3), di(hexachloro)−(R4), di(fluoro−,tetrachloro)−nitroisophthalamide (R5), and their complexes with C2H3O2−, C7H5O2−, NO3−, H2PO4−, and ClO4− oxoanions were computed and obtained using the density functional theory calculations at the B3LYP/6-31G(d,p) theoretical level in gas phase. According to the computed results, all of oxoanions can form the stable complexes with amide receptors R1−R5 via exothermic process in which receptor R1 is found to interact with oxoanions through hydrogen bonds whereas the receptors R2−R5 are found to interact with oxoanion through both of hydrogen and halogen bonds. It is clearly seen that acetate ion displays the strongest complexation interaction with all receptors compared to the other oxoanions. In addition, electronic properties of receptors R1−R5 in both gas and DMSO phases are modified after complexation with oxoanions. Therefore, the designed amide receptors may be potentially used for oxoanion sensing application.
硝基异酞酰胺受体的氧阴离子复合物:DFT 计算的启示
我们设计了酰胺衍生物受体,以研究其通过氢键和卤素键相互作用与氧阴离子络合的能力。硝基间苯二甲酰胺受体的结构、能量和电子特性,即二(苄基)-(R1)、二(六氟)-(R2)、二(氯、四氟)-(R3)、二(六氯)-(R4)、二(氟、四氯)-硝基间苯二甲酰胺(R5)及其与 C2H3O2-、C7H5O2-、NO、C7H5O2-、NO3-、H2PO4- 和 ClO4- 氧阳离子的配合物进行了气相 B3LYP/6-31G(d,p)理论水平的密度泛函理论计算。根据计算结果,所有氧阴离子都能通过放热过程与酰胺受体 R1-R5 形成稳定的配合物,其中受体 R1 通过氢键与氧阴离子相互作用,而受体 R2-R5 则通过氢键和卤素键与氧阴离子相互作用。可以清楚地看到,与其他氧阴离子相比,醋酸离子与所有受体的络合作用最强。此外,受体 R1-R5 在气相和 DMSO 相中的电子特性在与氧阴离子络合后都发生了改变。因此,所设计的酰胺受体有可能用于氧阴离子传感应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


