Design, synthesis and biological evaluation of novel diphenylamine analogues as NLRP3 inflammasome inhibitors
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
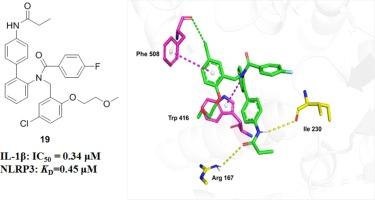
The aberrant activation of the NLRP3 inflammasome has been implicated in the pathogenesis of numerous inflammation-related diseases. Development of NLRP3 inflammasome inhibitors is expected to provide a new strategy for the treatment of these diseases. Herein, a novel series of diphenylamine derivatives were designed based on the lead compounds H20 and H28, and the preliminary structure–activity relationship was studied. The representative compound 19 displayed significantly higher inhibitory activity against NLRP3 inflammasome compared to lead compounds H20 and H28, with an IC50 of 0.34 μM. Mechanistic studies indicated that compound 19 directly targets the NLRP3 protein (KD: 0.45 μM), blocking the assembly and activation of the NLRP3 inflammasome, leading to anti-inflammatory effects and inhibition of cellular pyroptosis. Our findings indicated that compound 19 is a promising NLRP3 inhibitor and could potentially serve as a lead compound for further optimization.
作为 NLRP3 炎症小体抑制剂的新型二苯胺类似物的设计、合成和生物学评价
NLRP3 炎性体的异常激活与多种炎症相关疾病的发病机制有关。开发 NLRP3 炎性体抑制剂有望为治疗这些疾病提供一种新策略。本文以先导化合物 H20 和 H28 为基础,设计了一系列新型二苯胺衍生物,并对其结构-活性关系进行了初步研究。与先导化合物 H20 和 H28 相比,代表性化合物 19 对 NLRP3 炎性体的抑制活性明显更高,IC50 为 0.34 μM。机理研究表明,化合物 19 直接靶向 NLRP3 蛋白(KD:0.45 μM),阻断了 NLRP3 炎性体的组装和活化,从而产生抗炎作用并抑制细胞的脓毒症。我们的研究结果表明,化合物 19 是一种很有前景的 NLRP3 抑制剂,有可能成为进一步优化的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


