Monte Carlo-based simulation of virtual 3 and 4-dimensional cone-beam computed tomography from computed tomography images: An end-to-end framework and a deep learning-based speedup strategy
IF 3.4
Q2 ONCOLOGY
引用次数: 0
Abstract
Background and purpose:
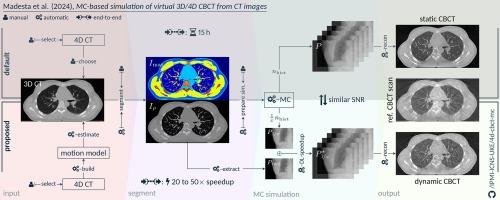
In radiotherapy, precise comparison of fan-beam computed tomography (CT) and cone-beam CT (CBCT) arises as a commonplace, yet intricate task. This paper proposes a publicly available end-to-end pipeline featuring an intrinsic deep-learning-based speedup technique for generating virtual 3D and 4D CBCT from CT images.
Materials and methods:
Physical properties, derived from CT intensity information, are obtained through automated whole-body segmentation of organs and tissues. Subsequently, Monte Carlo (MC) simulations generate CBCT X-ray projections for a full circular arc around the patient employing acquisition settings matched with a clinical CBCT scanner (modeled according to Varian TrueBeam specifications). In addition to 3D CBCT reconstruction, a 4D CBCT can be simulated with a fully time-resolved MC simulation by incorporating respiratory correspondence modeling. To address the computational complexity of MC simulations, a deep-learning-based speedup technique is developed and integrated that uses projection data simulated with a reduced number of photon histories to predict a projection that matches the image characteristics and signal-to-noise ratio of the reference simulation.
Results:
MC simulations with default parameter setting yield CBCT images with high agreement to ground truth data acquired by a clinical CBCT scanner. Furthermore, the proposed speedup technique achieves up to 20-fold speedup while preserving image features and resolution compared to the reference simulation.
Conclusion:
The presented MC pipeline and speedup approach provide an openly accessible end-to-end framework for researchers and clinicians to investigate limitations of image-guided radiation therapy workflows built on both (4D) CT and CBCT images.
基于蒙特卡罗的虚拟三维和四维锥形束计算机断层扫描模拟:端到端框架和基于深度学习的加速策略
背景和目的:在放射治疗中,精确比较扇形束计算机断层扫描(CT)和锥形束计算机断层扫描(CBCT)是一项常见但复杂的任务。本文提出了一种公开可用的端到端流水线,该流水线采用基于深度学习的内在加速技术,可从 CT 图像生成虚拟 3D 和 4D CBCT。随后,采用与临床 CBCT 扫描仪(根据瓦里安 TrueBeam 规格建模)相匹配的采集设置,通过蒙特卡罗(Monte Carlo,MC)模拟生成患者周围全圆弧的 CBCT X 射线投影。除了三维 CBCT 重建外,还可以通过结合呼吸对应建模,使用全时间分辨 MC 仿真模拟四维 CBCT。为了解决 MC 模拟的计算复杂性问题,我们开发并集成了一种基于深度学习的加速技术,该技术使用用较少光子历史记录模拟的投影数据来预测与参考模拟的图像特征和信噪比相匹配的投影结果:采用默认参数设置的 MC 模拟生成的 CBCT 图像与临床 CBCT 扫描仪获取的地面实况数据具有很高的一致性。结论:本文介绍的 MC 管道和加速方法为研究人员和临床医生提供了一个可公开访问的端到端框架,用于研究基于 (4D) CT 和 CBCT 图像的图像引导放射治疗工作流程的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physics and Imaging in Radiation Oncology
Physics and Astronomy-Radiation
CiteScore
5.30
自引率
18.90%
发文量
93
审稿时长
6 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


