Schleyer hyperconjugative aromaticity in indene scaffolds
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
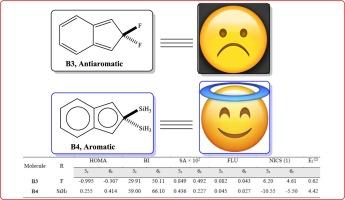
In the present research, the Schleyer hyperconjugative aromaticity was used to enhance the aromaticity of indene scaffolds. The first section of the research focused on examining the thermodynamic stability and electronic characteristics of the four structural isomers of indene. Results indicate that 1H-indene scaffolds are the most stable thermodynamic isomers of indene derivatives exceeding 100 kJ/mol. The hierarchy of stability is as follows: 1H-indenes > 2H-indenes > 5H-indenes > 4H-indenes. The aromaticity of 5- and 6-membered rings in the isomeric structures was investigated using the B3LYP/6–311 + G(d,p) method in the second section of the study. The hyperconjugative aromaticity of 5MR and 6MR was assessed using the harmonic oscillator model of aromaticity index, Bird index, Shannon index, aromatic fluctuation index, and the nucleus independent chemical shift. The results reveal that introducing electron-donating groups on 6MR enhances the aromaticity of 5MR. Moreover, adding two fluorine atoms on the Csp3 site negatively affects the aromaticity and induces antiaromaticity in the indene scaffold. Based on aromaticity data, the order of increasing aromaticity in the presence of different groups is F < CH3 < H < SiH3 < GeH3 < Si(CH3)3 < Ge(CH3)3. The extent of the Schleyer hyperconjugation interaction was also quantified using the E(2) parameter obtained from the NBO calculations.
茚支架中的 Schleyer 超共轭芳香性
本研究利用 Schleyer 超共轭芳香性来增强茚支架的芳香性。研究的第一部分重点考察了茚的四种结构异构体的热力学稳定性和电子特性。结果表明,1H-茚支架是茚衍生物中热力学稳定性最高的异构体,超过 100 kJ/mol。稳定性的等级划分如下1H-茚> 2H-茚> 5H-茚> 4H-茚。研究的第二部分采用 B3LYP/6-311 + G(d,p) 方法研究了异构体结构中 5 元环和 6 元环的芳香性。利用芳香指数、伯德指数、香农指数、芳香波动指数和核独立化学位移的谐振子模型评估了 5MR 和 6MR 的超共轭芳香性。结果表明,在 6MR 上引入电子供能基团会增强 5MR 的芳香性。此外,在 Csp3 位点上添加两个氟原子会对芳香性产生负面影响,并诱导茚支架产生反芳香性。根据芳香性数据,不同基团存在时芳香性增加的顺序是 F < CH3 < H < SiH3 < GeH3 < Si(CH3)3 < Ge(CH3)3。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


