Excess glucose alone induces hepatocyte damage due to oxidative stress and endoplasmic reticulum stress
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Type 2 diabetes mellitus (DM) is a significant risk factor for metabolic dysfunction-associated steatotic liver disease (MASLD) and hepatocellular carcinoma (HCC). With the increasing prevalence of type 2 DM and MASLD due to lifestyle changes, understanding their impact on liver health is crucial. However, the hepatocellular damage caused by glucose alone is unknown. This study investigates the effect of excess glucose on hepatocytes, focusing on oxidative stress, endoplasmic reticulum stress (ER stress), apoptosis, autophagy, and cell proliferation. We treated an immortalized-human hepatocyte cell line with excess glucose and analyzed. Excess glucose induced oxidative stress and ER stress in a time- and concentration-dependent manner, leading to apoptosis. Oxidative stress and ER stress were independently induced by excess glucose. Proteasome inhibitors and palmitic acid exacerbated glucose-induced stress, leading to the formation of Mallory-Denk body-like inclusion bodies. Despite these stresses, autophagic flux was not altered. Excess glucose also caused DNA damage but did not affect cell proliferation. This suggests that glucose itself can contribute to the progression of metabolic dysfunction-associated steatohepatitis (MASH) and carcinogenesis of HCC in patients with type 2 DM. Managing blood glucose levels is crucial to prevent hepatocyte damage and associated complications.
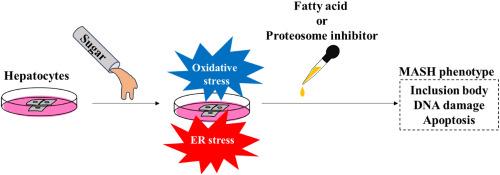
由于氧化应激和内质网应激,仅过量葡萄糖就会诱发肝细胞损伤。
2 型糖尿病(DM)是代谢功能障碍相关性脂肪性肝病(MASLD)和肝细胞癌(HCC)的重要危险因素。随着生活方式的改变,2 型糖尿病和代谢性脂肪肝的发病率不断上升,了解它们对肝脏健康的影响至关重要。然而,仅由葡萄糖引起的肝细胞损伤尚不清楚。本研究调查了过量葡萄糖对肝细胞的影响,重点关注氧化应激、内质网应激(ER应激)、细胞凋亡、自噬和细胞增殖。我们用过量葡萄糖处理了永生化人肝细胞系,并对其进行了分析。过量葡萄糖以时间和浓度依赖的方式诱导氧化应激和ER应激,导致细胞凋亡。过量葡萄糖可独立诱导氧化应激和ER应激。蛋白酶体抑制剂和棕榈酸加剧了葡萄糖诱导的应激,导致马洛里-登克体样包涵体的形成。尽管存在这些应激,自噬通量并没有改变。过量的葡萄糖也会造成 DNA 损伤,但不会影响细胞增殖。这表明,葡萄糖本身可导致 2 型糖尿病患者代谢功能障碍相关性脂肪性肝炎(MASH)和 HCC 癌变的进展。控制血糖水平对于预防肝细胞损伤和相关并发症至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


