IBR1, a novel endogenous IFIH1-binding dsRNA, governs IFIH1 activation and M1 macrophage polarisation in ARDS
Abstract
Background
Uncontrolled inflammation caused by macrophages and monocytes plays a crucial role in worsening acute respiratory distress syndrome (ARDS). Previous studies have highlighted the importance of IFIH1 in regulating macrophage polarisation in ARDS triggered by pneumonia. However, the mechanisms by which IFIH1 is activated in ARDS remain unclear.
Methods
In this study, we utilised multiomics sequencing and molecular interaction experiments to explore the molecular mechanisms underlying IFIH1 activation in ARDS. Through the use of conditional gene knockout mice and primary cells, we demonstrated the significant role of these mechanisms in the development of ARDS. Additionally, we validated the associations between these mechanisms and ARDS by quantitative PCR analysis of CD14+ cells obtained from the peripheral blood of 140 ARDS patients.
Results
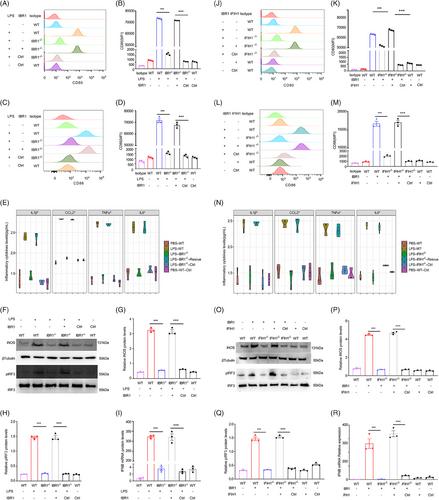
Our investigation revealed that lipopolysaccharide, a critical component derived from Gram-negative bacteria, activated IFIH1 by upregulating a novel transcript known as IFIH1-binding RNA1 (IBR1) in monocytes and macrophages. Specifically, as an endogenous double-stranded RNA, IBR1 bind to the helicase domain of IFIH1 because of its unique double-stranded structure. Deletion of IBR1 significantly reduced the activation of IFIH1, M1 polarisation of macrophages, and inflammatory lung injury in ARDS. Moreover, IBR1 directly induced M1 polarisation of macrophages and ARDS, whereas deletion of IFIH1 inhibited IBR1-induced macrophage M1 polarisation and inflammatory lung injury. Importantly, we observed a notable increase in IBR1 expression in ARDS patients with pneumonia caused by Gram-negative bacteria. Furthermore, we demonstrated that the delivery of IFIH1 mutants through exosomes effectively counteracted IBR1, thereby reducing pulmonary inflammation and alleviating lung injury.
Conclusions
This study revealed a novel mechanism involving IBR1, an endogenous double-stranded RNA (dsRNA) that binds to IFIH1, shedding light on the complex process of macrophage polarisation in ARDS. The administration of IFIH1 variants has the potential to eliminate pulmonary dsRNA and alleviate inflammatory lung injury in ARDS.
Highlights
-
In monocytes and macrophages, the endogenous double-stranded RNA, IFIH1-binding RNA 1 (IBR1), binds to the helicase domain of IFIH1 because of its unique double-stranded structure.
-
IBR1 plays a significant role in macrophage polarisation and the development of acute respiratory distress syndrome (ARDS) induced by Gram-negative bacteria or lipopolysaccharide (LPS).
-
Administration of IFIH1 variants has potential for eliminating pulmonary IBR1 and reducing inflammatory lung injury in ARDS patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


