Tumor-infiltrating immune cell profiles and changes associate with additional trastuzumab in preoperative chemotherapy for patients with HER2-positive gastric cancer
IF 6.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
HER2(+) gastric cancer (GC) can benefit from trastuzumab. However, the impact of additional trastuzumab in preoperative treatment on immune cells remains largely unknown. In cohort I, immune cells were detected by immunohistochemistry in 1321 patients. Then 88 HER2(+) patients received preoperative therapy were collected as cohort II. Immune cell profiles and changes were analyzed in paired pre- and post-operative specimens using multiple immunohistochemistry staining. In the treatment-naive GC patients (n = 1002), CD3+ and CD8+ T cell infiltration was significantly lower in the HER2(+) GC patients together with higher FoxP3+ T cells compared with HER2(−). However, FoxP3+ T and CD20+ B cell infiltration was significantly higher in HER2(+) GC after neoadjuvant chemotherapy (n = 319). The trastuzumab-exposed group had higher CD8+ T and lower FoxP3+ T cell infiltration and CD8+ T cell was even more significant in responders. Additionally, tertiary lymphoid structure (TLS) density increased in invasion margin of residual tumors. Patients with lower TLS in the tumor core or lower FoxP3+ T cells had better overall survival in the trastuzumab-exposed group. Addition of trastuzumab modulates the immune microenvironment, suggesting the potential mechanism of the favorable outcome of anti-HER2 therapy and providing a theoretical rationale for the combinational immunotherapy in resectable HER2(+) GC patients.
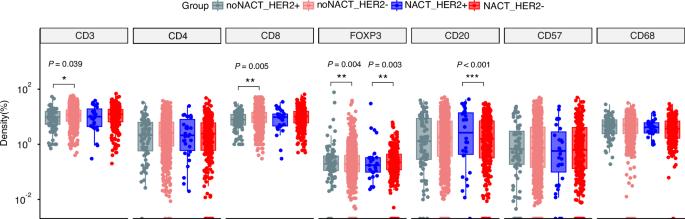
HER2 阳性胃癌患者术前化疗中额外使用曲妥珠单抗的肿瘤浸润免疫细胞特征及其变化。
背景HER2(+)胃癌(GC)可从曲妥珠单抗中获益。然而,术前治疗中额外使用曲妥珠单抗对免疫细胞的影响在很大程度上仍是未知数:方法:在队列 I 中,通过免疫组化检测了 1321 例患者的免疫细胞。然后收集 88 例接受术前治疗的 HER2(+)患者作为队列 II。采用多重免疫组化染色法分析了术前和术后配对标本中免疫细胞的特征和变化:在未经治疗的 GC 患者(n = 1002)中,与 HER2(-)相比,HER2(+)GC 患者的 CD3+ 和 CD8+ T 细胞浸润明显较低,FoxP3+ T 细胞也较高。然而,新辅助化疗后(n = 319),HER2(+)GC 患者的 FoxP3+ T 细胞和 CD20+ B 细胞浸润明显升高。曲妥珠单抗暴露组的 CD8+ T 细胞浸润较高,而 FoxP3+ T 细胞浸润较低,CD8+ T 细胞在应答者中的浸润更为明显。此外,残留肿瘤浸润边缘的三级淋巴结构(TLS)密度增加。肿瘤核心TLS较低或FoxP3+ T细胞较少的患者在曲妥珠单抗暴露组的总生存率更高:加入曲妥珠单抗可调节免疫微环境,提示了抗HER2治疗取得良好疗效的潜在机制,并为可切除HER2(+)GC患者的联合免疫疗法提供了理论依据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


