Monocyte and macrophage profiles in patients with inherited long-chain fatty acid oxidation disorders
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-09-20
DOI:10.1016/j.bbadis.2024.167524
引用次数: 0
Abstract
Patients with inherited disorders of the long-chain fatty acid oxidation (lcFAO) machinery present with a heterogeneous profile of disease manifestations and aggravation of symptoms is often triggered by inflammatory activation. Monocytes and macrophages are innate immune cells that play a major role in the onset and resolution of inflammation. These cells undergo metabolic rewiring upon activation including the regulation of the FAO rate. The rewiring of FAO and the effect of lcFAO disorders (lcFAOD) on human monocyte and macrophage phenotype and function remain largely unknown. Here, we performed extensive phenotyping of circulating monocytes and analyzed plasma cytokine levels in 11 lcFAOD patients and 11 matched control subjects. In patients with lcFAOD, we observed induced plasma levels of the inflammatory cytokines IL-1β and IL-6, and enhanced CD206 and CD62L surface marker expression in circulating monocyte subsets. To mimic the most common lcFAOD very-long-chain acyl-CoA dehydrogenase disorder (VLCADD), we used siRNA-mediated knockdown of the ACADVL gene (encoding VLCAD) in macrophages derived from healthy volunteers. Hereby, we found that siVLCAD affected IL-4-induced alternative macrophage activation while leaving LPS responses and cellular metabolism intact. In the same line, monocyte-derived macrophages from lcFAOD patients had elevated levels of the IL-4-induced alternative macrophage markers CD206 and CD200R. Still, they did not show major metabolic defects or changes in the LPS-induced inflammatory response. Our results indicate that monocytes and macrophages from lcFAOD patients present no major inflammatory or metabolic differences and show that IL-4-induced surface markers are intertwined with lcFAO in human macrophages.
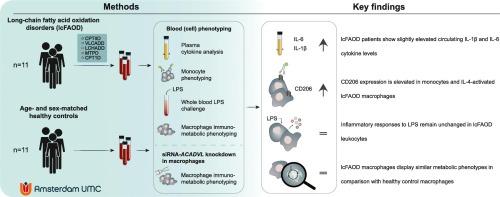
遗传性长链脂肪酸氧化紊乱患者的单核细胞和巨噬细胞概况。
长链脂肪酸氧化(lcFAO)机制遗传性疾病患者的疾病表现各不相同,炎症激活往往会引发症状加重。单核细胞和巨噬细胞是先天性免疫细胞,在炎症的发生和消退过程中发挥着重要作用。这些细胞在激活后会进行新陈代谢的重新布线,包括调节 FAO 的速率。FAO 的重新布线以及 lcFAO 紊乱(lcFAOD)对人类单核细胞和巨噬细胞表型和功能的影响在很大程度上仍是未知的。在这里,我们对 11 名 lcFAOD 患者和 11 名匹配的对照组受试者的循环单核细胞进行了广泛的表型分析,并对血浆细胞因子水平进行了分析。在 lcFAOD 患者中,我们观察到血浆中的炎性细胞因子 IL-1β 和 IL-6 水平升高,循环单核细胞亚群的 CD206 和 CD62L 表面标志物表达增强。为了模拟最常见的 lcFAOD 极长链酰基-CoA 脱氢酶紊乱症(VLCADD),我们使用 siRNA 介导敲除健康志愿者巨噬细胞中的 ACADVL 基因(编码 VLCAD)。因此,我们发现 siVLCAD 会影响 IL-4 诱导的巨噬细胞替代活化,而 LPS 反应和细胞代谢则保持不变。同样,lcFAOD 患者单核细胞衍生的巨噬细胞中,IL-4 诱导的替代性巨噬细胞标记物 CD206 和 CD200R 水平升高。不过,它们并没有表现出重大的代谢缺陷或 LPS 诱导的炎症反应的变化。我们的研究结果表明,lcFAOD 患者的单核细胞和巨噬细胞在炎症或新陈代谢方面没有重大差异,并表明 IL-4 诱导的表面标志物与人类巨噬细胞中的 lcFAO 相互交织。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


