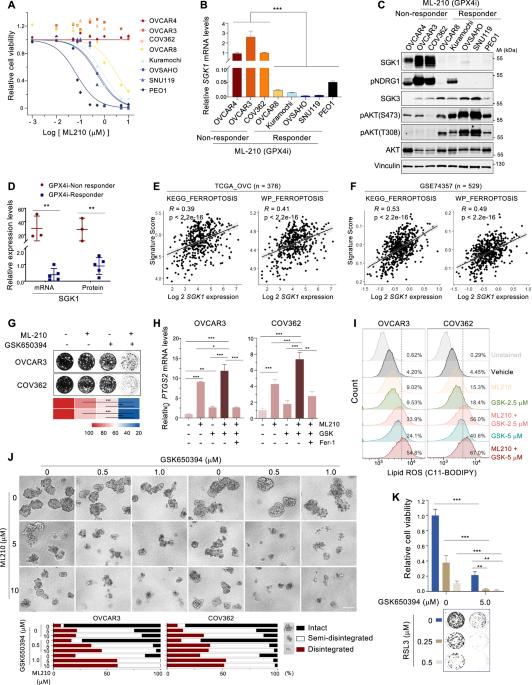
SGK1 suppresses ferroptosis in ovarian cancer via NRF2-dependent and -independent pathways
IF 7.3
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
High-grade serous ovarian cancer (HGSOC) is a highly aggressive disease often developing resistance to current therapies, necessitating new treatment strategies. Our study identifies SGK1, a key effector in the PI3K pathway, as a promising therapeutic target to exploit ferroptosis, a distinct form of cell death induced by iron overload and lipid peroxidation. Importantly, SGK1 activation, whether through high expression or the constitutively active SGK1-S422D mutation, confers resistance to ferroptosis in HGSOC. Conversely, SGK1 inhibition significantly enhances sensitivity to ferroptosis, as shown by increased PTGS2 expression (a ferroptosis marker), lipid peroxidation, and toxic-free iron levels. Remarkably, this enhanced cytotoxicity is reversed by ferrostatin-1 and the iron chelator deferoxamine, highlighting the pivotal roles of lipid peroxidation and iron dysregulation in the process. Mechanistically, SGK1 protects HGSOC cells from ferroptosis via NRF2-dependent pathways, promoting glutathione synthesis and iron homeostasis, and NRF2-independent pathways via mTOR/SREBP1/SCD1-mediated lipogenesis. Notably, pharmacological SGK1 inhibition sensitizes HGSOC xenograft models to ferroptosis induction, highlighting its therapeutic potential. These findings establish SGK1 as a critical regulator of ferroptosis and suggest targeting SGK1 alongside ferroptosis pathways as a potential therapeutic strategy for HGSOC patients.
SGK1 通过 NRF2 依赖性和非依赖性途径抑制卵巢癌中的铁突变。
高分化浆液性卵巢癌(HGSOC)是一种侵袭性很强的疾病,往往会对目前的疗法产生抗药性,因此需要新的治疗策略。我们的研究发现,PI3K 通路中的一个关键效应因子 SGK1 是一种很有前景的治疗靶点,可用于利用铁中毒(一种由铁超载和脂质过氧化诱导的独特细胞死亡形式)。重要的是,无论是通过高表达还是组成型活性 SGK1-S422D 突变,SGK1 的活化都赋予了 HGSOC 对铁中毒的抵抗力。相反,抑制 SGK1 会显著增强对铁变态反应的敏感性,这表现在 PTGS2 表达(铁变态反应标志物)、脂质过氧化和无毒铁水平的增加。值得注意的是,这种增强的细胞毒性可被铁前列素-1 和铁螯合剂去铁胺逆转,突出了脂质过氧化和铁失调在这一过程中的关键作用。从机理上讲,SGK1 可通过 NRF2 依赖性途径(促进谷胱甘肽合成和铁稳态)和 NRF2 非依赖性途径(mTOR/SREBP1/SCD1 介导的脂肪生成)保护 HGSOC 细胞免于铁变态反应。值得注意的是,药理抑制 SGK1 可使 HGSOC 异种移植模型对铁变态反应诱导敏感,从而突出了其治疗潜力。这些发现确定了 SGK1 是铁蛋白沉积的关键调节因子,并建议将 SGK1 与铁蛋白沉积通路一起作为 HGSOC 患者的潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


