Beyond glucose: The crucial role of redox signaling in β-cell metabolic adaptation
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
Redox signaling mediated by reversible oxidative cysteine thiol modifications is crucial for driving cellular adaptation to dynamic environmental changes, maintaining homeostasis, and ensuring proper function. This is particularly critical in pancreatic β-cells, which are highly metabolically active and play a specialized role in whole organism glucose homeostasis. Glucose stimulation in β-cells triggers signals leading to insulin secretion, including changes in ATP/ADP ratio and intracellular calcium levels. Additionally, lipid metabolism and reactive oxygen species (ROS) signaling are essential for β-cell function and health.
Methods
We employed IodoTMT isobaric labeling combined with tandem mass spectrometry to elucidate redox signaling pathways in pancreatic β-cells.
Results
Glucose stimulation significantly increases ROS levels in β-cells, leading to targeted reversible oxidation of proteins involved in key metabolic pathways such as glycolysis, the tricarboxylic acid (TCA) cycle, pyruvate metabolism, oxidative phosphorylation, protein processing in the endoplasmic reticulum (ER), and insulin secretion. Furthermore, the glucose-induced increase in reversible cysteine oxidation correlates with the presence of other post-translational modifications, including acetylation and phosphorylation.
Conclusions
Proper functioning of pancreatic β-cell metabolism relies on fine-tuned regulation, achieved through a sophisticated system of diverse post-translational modifications that modulate protein functions. Our findings demonstrate that glucose induces the production of ROS in pancreatic β-cells, leading to targeted reversible oxidative modifications of proteins. Furthermore, protein activity is modulated by acetylation and phosphorylation, highlighting the complexity of the regulatory mechanisms in β-cell function.
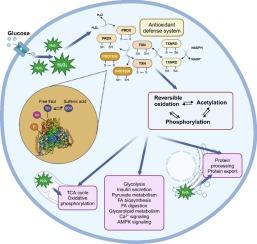
超越葡萄糖:氧化还原信号在β细胞代谢适应中的关键作用。
目的:由可逆氧化半胱氨酸巯基修饰介导的氧化还原信号对于推动细胞适应动态环境变化、维持体内平衡和确保正常功能至关重要。这一点对胰腺β细胞尤为重要,因为β细胞代谢高度活跃,在整个机体的葡萄糖稳态中发挥着特殊作用。β细胞中的葡萄糖刺激会触发导致胰岛素分泌的信号,包括 ATP/ADP 比率和细胞内钙水平的变化。此外,脂质代谢和活性氧(ROS)信号对β细胞的功能和健康也至关重要:方法:我们采用 IodoTMT 等位标记结合串联质谱法来阐明胰腺 β 细胞的氧化还原信号通路:结果:葡萄糖刺激可明显增加β细胞中的ROS水平,导致参与糖酵解、三羧酸循环、丙酮酸代谢、氧化磷酸化、内质网(ER)中的蛋白质加工和胰岛素分泌等关键代谢途径的蛋白质发生定向可逆氧化。此外,葡萄糖诱导的可逆半胱氨酸氧化增加与其他翻译后修饰(包括乙酰化和磷酸化)的存在相关:胰岛β细胞新陈代谢的正常运行依赖于微调,而微调是通过调节蛋白质功能的各种翻译后修饰的复杂系统实现的。我们的研究结果表明,葡萄糖会诱导胰岛β细胞产生 ROS,从而导致蛋白质发生有针对性的可逆氧化修饰。此外,蛋白质的活性还受到乙酰化和磷酸化的调节,凸显了β细胞功能调节机制的复杂性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


