A novel encapsulation approach to enhance the delivery and antitumor activity of docetaxel in breast cancer therapy
IF 3.7
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Docetaxel (DTX) is one of the most potent anticancer drugs but its extensive side effects necessitate innovative formulations. In this study, we aimed to investigate the expression pattern of apoptotic proteins, cell cycle arrest, and apoptosis induction after treatment with encapsulated DTX in alginate-chitosan nanoparticles in both breast cancer cells (MCF-7) and peripheral blood mononuclear cells (PBMCs). The characterization of the nanoparticles revealed a spherical shape with a size <50 nm, a hydrodynamic diameter of 200 nm, a Polydispersity Index of 0.5, and an encapsulation efficiency of 98.75 %. The free drug was released completely within 11 h while encapsulated DTX was released only 34 % in 96 h. The encapsulated drug indicated higher cytotoxicity on MCF-7 cells and the half inhibitory concentration (IC50) value was 2 µg/ml after 72 h. Quantitative real-time PCR demonstrated a significant increase in cell death as the expression of apoptosis regulatory protein (Bcl-2) was downregulated with no impact on Bax in the MCF-7 cells. A notable decrease in the expression pattern of pro-inflammatory cytokine (IL-1β) in PBMCs indicated less inflammation induction. Flow cytometry analysis revealed that the newly formulated drug induced less opoptosis in PBMCs than the free DTX. Cell cycle arrest in the sub-G1 phase was observed for the free drug while the encapsulated drug exhibited no significant changes. Our results suggest the high toxicity of the formulated drug in contrast to the free DTX on the MCF-7 cell line, minimal blood cell side effects, and no inflammation positioning it as a promising alternative to free docetaxel.
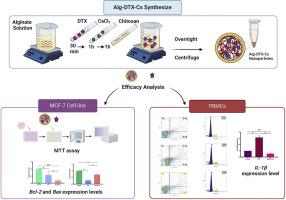
在乳腺癌治疗中增强多西他赛给药和抗肿瘤活性的新型封装方法
多西他赛(DTX)是最有效的抗癌药物之一,但它的副作用很大,因此需要创新配方。在这项研究中,我们的目的是研究在乳腺癌细胞(MCF-7)和外周血单核细胞(PBMCs)中使用海藻酸盐-壳聚糖封装的 DTX 纳米颗粒后,凋亡蛋白的表达模式、细胞周期停滞和凋亡诱导。实时定量 PCR 显示,MCF-7 细胞中细胞凋亡调节蛋白(Bcl-2)的表达下调,而 Bax 的表达没有受到影响,因此细胞死亡明显增加。促炎细胞因子(IL-1β)在 PBMCs 中的表达模式明显下降,表明炎症诱导减少。流式细胞术分析表明,与游离 DTX 相比,新配制的药物在 PBMCs 中诱导的细胞凋亡更少。游离药物的细胞周期停滞在亚 G1 期,而封装药物则无明显变化。我们的研究结果表明,与游离 DTX 相比,新配制的药物对 MCF-7 细胞株的毒性较高,对血液细胞的副作用最小,而且不会引起炎症,因此有望成为游离多西他赛的替代品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.30
自引率
13.20%
发文量
367
审稿时长
33 days
期刊介绍:
The Journal of Pharmaceutical Sciences will publish original research papers, original research notes, invited topical reviews (including Minireviews), and editorial commentary and news. The area of focus shall be concepts in basic pharmaceutical science and such topics as chemical processing of pharmaceuticals, including crystallization, lyophilization, chemical stability of drugs, pharmacokinetics, biopharmaceutics, pharmacodynamics, pro-drug developments, metabolic disposition of bioactive agents, dosage form design, protein-peptide chemistry and biotechnology specifically as these relate to pharmaceutical technology, and targeted drug delivery.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


