BRD9 promotes the progression of gallbladder cancer via CST1 upregulation and interaction with FOXP1 through the PI3K/AKT pathway and represents a therapeutic target
IF 4.5
3区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
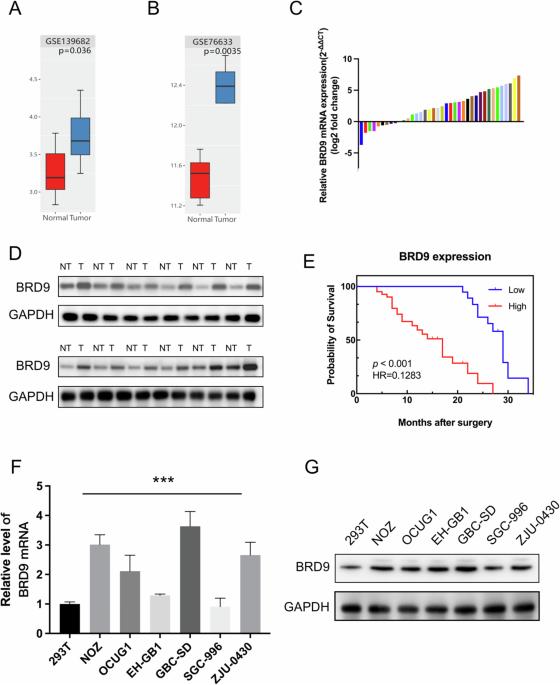
Gallbladder cancer (GBC) is highly aggressive and has poor prognosis, with most patients only diagnosed at an advanced stage. Furthermore, treatment options are limited, and their effect is unsatisfactory. Bromodomain-containing protein (BRD) is an epigenetic regulator that plays a carcinogenic role in several tumors, including squamous cell lung cancer, acute myeloid leukemia, synovial sarcoma, and malignant rhabdomyosarcoma. However, the expression, biological function, and molecular mechanisms of action of BRD9 in GBC are still unknown. Kaplan–Meier analysis, qRT-PCR, and analysis of clinical features were used to assess the clinical significance of BRD9 in GBC. Cell Counting Kit-8 and colony formation assays were performed to determine the effects of BRD9 on cell growth. The functional role of BRD9 in GBC was explored using qRT-PCR, western blotting, siRNA, and CHIP-qPCR. mRNA sequencing was performed to explore the underlying mechanisms of BRD9, and a nude mouse model of GBC was established to explore the anti-tumor effects of the BRD9 inhibitor I-BRD9 in vivo. BRD9 expression was elevated in GBC tissues compared with adjacent non-tumor tissues, and high BRD9 expression was associated with poor prognosis in patients with GBC. BRD9 knockdown by siRNA significantly decreased cell growth. Targeting BRD9 with I-BRD9 inhibited the proliferation of GBC cells without significant toxic effects. Additionally, I-BRD9 treatment suppressed CST1 expression in GBC cell lines, thereby inhibiting the PI3K-AKT pathway. The transcription factor FOXP1 was found to interact with BRD9 to regulate CST1 expression. Collectively, these results suggest that BRD9 may be a promising biomarker and therapeutic target for GBC.
BRD9 通过 CST1 上调和与 FOXP1 的相互作用,通过 PI3K/AKT 通路促进胆囊癌的进展,是一个治疗靶点。
胆囊癌(GBC)侵袭性强,预后较差,大多数患者在晚期才被确诊。此外,治疗方案有限,效果也不理想。含溴结构域蛋白(Bromodomain-containing protein,BRD)是一种表观遗传调控因子,在多种肿瘤中起致癌作用,包括鳞状细胞肺癌、急性髓性白血病、滑膜肉瘤和恶性横纹肌肉瘤。然而,BRD9在GBC中的表达、生物学功能和分子作用机制仍然未知。我们采用卡普兰-梅耶分析、qRT-PCR和临床特征分析来评估BRD9在GBC中的临床意义。为了确定BRD9对细胞生长的影响,还进行了细胞计数试剂盒-8和集落形成试验。利用qRT-PCR、Western印迹、siRNA和CHIP-qPCR等方法探讨了BRD9在GBC中的功能作用,并进行了mRNA测序以探讨BRD9的潜在机制,同时建立了GBC裸鼠模型以探讨BRD9抑制剂I-BRD9在体内的抗肿瘤作用。与邻近的非肿瘤组织相比,BRD9在GBC组织中的表达升高,BRD9的高表达与GBC患者的不良预后有关。通过 siRNA 敲除 BRD9 能显著降低细胞生长。用I-BRD9靶向BRD9可抑制GBC细胞的增殖,且无明显毒副作用。此外,I-BRD9还能抑制GBC细胞系中CST1的表达,从而抑制PI3K-AKT通路。研究发现转录因子 FOXP1 与 BRD9 相互作用,调控 CST1 的表达。总之,这些结果表明,BRD9 可能是一种很有前景的 GBC 生物标记物和治疗靶标。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gene Therapy
医学-生化与分子生物学
CiteScore
9.70
自引率
2.00%
发文量
67
审稿时长
4-8 weeks
期刊介绍:
Gene Therapy covers both the research and clinical applications of novel therapeutic techniques based on a genetic component. Over the last few decades, significant advances in technologies ranging from identifying novel genetic targets that cause disease through to clinical studies, which show therapeutic benefit, have elevated this multidisciplinary field to the forefront of modern medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


