In vivo biomarkers of GABAergic function in epileptic rats treated with the GAT-1 inhibitor E2730
Abstract
Objective
E2730, an uncompetitive γ-aminobutyric acid (GABA) transporter-1 (GAT-1) inhibitor, has potent anti-seizure effects in a rodent model of chronic temporal lobe epilepsy, the kainic acid status epilepticus (KASE) rat model. In this study, we examined purported neuroimaging and physiological surrogate biomarkers of the effect of E2730 on brain GABAergic function.
Methods
We conducted a randomized cross-over study, incorporating 1-week treatments with E2730 (100 mg/kg/day subcutaneous infusion) or vehicle in epileptic post-KASE rats. KASE rats underwent serial 9.4 T magnetic resonance spectroscopy (MRS) measuring GABA and other brain metabolites, [18F]Flumazenil positron emission tomography (PET) quantifying GABAA receptor availability, quantitative electroencephalography (qEEG) and transcranial magnetic stimulation (TMS)–mediated motor activity, as well as continuous video-EEG recording to measure spontaneous seizures during each treatment. Age-matched, healthy control animals treated with E2730 or vehicle were also studied.
Results
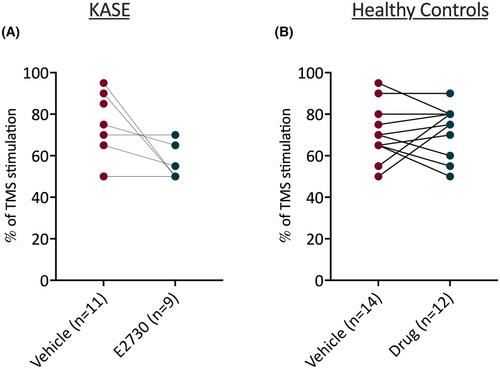
E2730 treatment significantly reduced spontaneous seizures, with 8 of 11 animals becoming seizure-free. MRS revealed that E2730-treated animals had significantly reduced taurine levels. [18F]Flumazenil PET imaging revealed no changes in GABA receptor affinity or density during E2730 treatment. The power of gamma frequency oscillations in the EEG was decreased significantly in the auditory cortex and hippocampus of KASE and control rats during E2730 treatment. Auditory evoked gamma frequency power was enhanced by E2730 treatment in the auditory cortex of KASE and healthy controls, but only in the hippocampus of KASE rats. E2730 did not influence motor evoked potentials triggered by TMS.
Significance
This study identified clinically relevant changes in multimodality imaging and functional purported biomarkers of GABAergic activity during E2730 treatment in epileptic and healthy control animals. These biomarkers could be utilized in clinical trials of E2730 and potentially other GABAergic drugs to provide surrogate endpoints, thereby reducing the cost of such trials.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


