Chronic stress promotes non-small cell lung cancer (NSCLC) progression through circMBOAT2 upregulation mediated by CTCF
IF 4.8
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Circular RNA (circRNA) has been demonstrated to play a pivotal role in tumor development. This study aimed to investigate the regulatory mechanism of circMBOAT2 in non-small cell lung cancer (NSCLC) and its association with tumor growth induced by chronic stress. We constructed stably transfected A549 and H1299 cell lines with circMBOAT2 overexpression and knockdown. Colony formation, scratch healing, Transwell and CCK-8 assays were conducted to evaluate the effects of circMBOAT2 in the presence or absence of norepinephrine (NE) treatment on the proliferation, migration, and invasion of NSCLC cells, respectively. Additionally, A chronic unpredictable mild stress (CUMS)-induced depression with heterotopic transplantation LLC and injection of antisense oligonucleotides (ASOs) targeting circMBOAT2 mouse model was established to evaluate the effect of chronic stress on tumorigenesis via circMBOAT2. Moreover, we investigated the regulatory effect of CCCTC binding factor (CTCF) on circMBOAT2 expression through in vivo and in vitro silencing of CTCF. Our results revealed a significant upregulation of circMBOAT2 in NSCLC cell lines and tumor tissues. circMBOAT2 knockdown inhibited the proliferation, migration, and invasion of NSCLC cells, while NE treatment reversed the cell suppression effect caused by circMBOAT2 knockdown. Notably, CUMS promoted tumor growth, while silencing circMBOAT2 inhibited tumor growth in vivo. Furthermore, we identified CTCF as the upstream regulator of circMBOAT2, which exhibited upregulation in NSCLC cells and tissues. Knockdown of CTCF reversed the promotional effect of CUMS on circMBOAT2 expression and tumor growth. Our findings provide evidence that CTCF mediates chronic stress in promoting of NSCLC progression through circMBOAT2. circMBOAT2 may serve as a potential biomarker and therapeutic target for NSCLC as well as the treatment of comorbid depression in NSCLC patients.
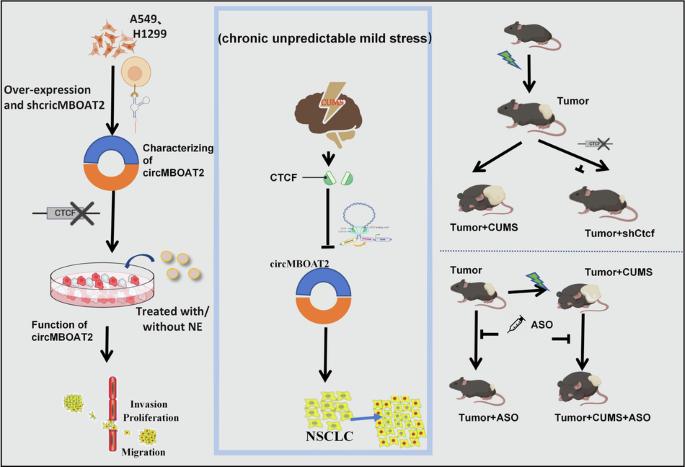
慢性压力通过 CTCF 介导的 circMBOAT2 上调促进非小细胞肺癌(NSCLC)的进展。
环状 RNA(circRNA)已被证明在肿瘤发生发展中起着关键作用。本研究旨在探讨circMBOAT2在非小细胞肺癌(NSCLC)中的调控机制及其与慢性应激诱导的肿瘤生长的关系。我们构建了稳定转染过表达和敲除 circMBOAT2 的 A549 和 H1299 细胞系。通过集落形成、划痕愈合、Transwell和CCK-8试验,分别评估了去甲肾上腺素(NE)存在或不存在时circMBOAT2对NSCLC细胞增殖、迁移和侵袭的影响。此外,我们还建立了一个慢性不可预测轻度应激(CUMS)诱导的抑郁症异位移植小鼠模型,并注射了针对 circMBOAT2 的反义寡核苷酸(ASO),以评估慢性应激通过 circMBOAT2 对肿瘤发生的影响。此外,我们还通过体内和体外沉默 CTCF,研究了 CCCTC 结合因子(CTCF)对 circMBOAT2 表达的调控作用。我们的研究结果表明,circMBOAT2在NSCLC细胞系和肿瘤组织中明显上调,circMBOAT2敲除可抑制NSCLC细胞的增殖、迁移和侵袭,而NE处理可逆转circMBOAT2敲除对细胞的抑制作用。值得注意的是,CUMS 促进了肿瘤的生长,而沉默 circMBOAT2 则抑制了肿瘤在体内的生长。此外,我们还发现 CTCF 是 circMBOAT2 的上游调节因子,它在 NSCLC 细胞和组织中表现出上调。敲除 CTCF 可逆转 CUMS 对 circMBOAT2 表达和肿瘤生长的促进作用。我们的研究结果提供了 CTCF 通过 circMBOAT2 介导慢性应激促进 NSCLC 进展的证据。circMBOAT2 可作为 NSCLC 的潜在生物标记物和治疗靶点,也可用于治疗 NSCLC 患者合并的抑郁症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


