FABP4-mediated lipid metabolism promotes TNBC progression and breast cancer stem cell activity
IF 9.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Metabolic remodeling is a pivotal feature of cancer, with cancer stem cells frequently showcasing distinctive metabolic behaviors. Nonetheless, understanding the metabolic intricacies of triple-negative breast cancer (TNBC) and breast cancer stem cells (BCSCs) has remained elusive. In this study, we meticulously characterized the metabolic profiles of TNBC and BCSCs and delved into their potential implications for TNBC treatment. Our findings illuminated the robust lipid metabolism activity within TNBC tumors, especially in BCSCs. Furthermore, we discovered that Fabp4, through its mediation of fatty acid uptake, plays a crucial role in regulating TNBC lipid metabolism. Knocking down Fabp4 or inhibiting its activity significantly suppressed TNBC tumor progression in both the MMTV-Wnt1 spontaneous TNBC model and the TNBC patient-derived xenograft model. Mechanistically, Fabp4's influence on TNBC tumor progression was linked to its regulation of mitochondrial stability, the CPT1-mediated fatty acid oxidation process, and ROS production. Notably, in a high-fat diet model, Fabp4 deficiency proved to be a substantial inhibitor of obesity-accelerated TNBC progression. Collectively, these findings shed light on the unique metabolic patterns of TNBC and BCSCs, underscore the biological significance of Fabp4-mediated fatty acid metabolism in governing TNBC progression, and offer a solid theoretical foundation for considering metabolic interventions in breast cancer treatment.
Significance
Triple-negative breast cancer progression and breast cancer stem cell activity can be restricted by targeting a critical regulator of lipid responses, FABP4.
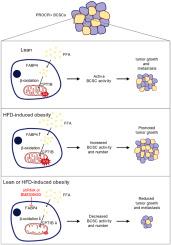
FABP4 介导的脂质代谢促进 TNBC 的进展和乳腺癌干细胞的活性。
代谢重塑是癌症的一个关键特征,癌症干细胞经常表现出与众不同的代谢行为。然而,人们对三阴性乳腺癌(TNBC)和乳腺癌干细胞(BCSCs)复杂的新陈代谢过程的了解仍然很有限。在这项研究中,我们仔细描述了 TNBC 和 BCSCs 的代谢特征,并深入研究了它们对 TNBC 治疗的潜在影响。我们的研究结果揭示了 TNBC 肿瘤内,尤其是 BCSCs 中强大的脂质代谢活性。此外,我们还发现,Fabp4通过介导脂肪酸摄取,在调控TNBC脂质代谢中发挥着至关重要的作用。在MMTV-Wnt1自发TNBC模型和TNBC患者异种移植模型中,敲除Fabp4或抑制其活性都能显著抑制TNBC肿瘤的进展。从机理上讲,Fabp4对TNBC肿瘤进展的影响与其对线粒体稳定性、CPT1介导的脂肪酸氧化过程和ROS产生的调控有关。值得注意的是,在高脂饮食模型中,Fabp4 的缺乏被证明是肥胖加速 TNBC 进展的一个重要抑制因素。总之,这些发现揭示了 TNBC 和 BCSCs 独特的代谢模式,强调了 Fabp4 介导的脂肪酸代谢在控制 TNBC 进展中的生物学意义,并为考虑乳腺癌治疗中的代谢干预提供了坚实的理论基础。意义:通过靶向脂质反应的关键调节因子FABP4,可以限制三阴性乳腺癌的进展和乳腺癌干细胞的活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


