Dual-modified antisense oligonucleotides targeting oncogenic protocadherin to treat gastric cancer
IF 6.4
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
The objective of this study was to develop an innovative treatment strategy utilizing antisense oligonucleotides (ASOs) that target the gene encoding protocadherin alpha 11 (PCDHA11) and to elucidate the role of PCDHA11 in gastric cancer cells. We designed and screened 54 amido-bridged nucleic acid (AmNA)-modified ASOs, selecting them based on PCDHA11-knockdown efficacy, in vitro and in vivo activity, and off-target effects. We assessed the impact of AmNA-modified anti-PCDHA11 ASOs on cellular functions and signaling pathways, and investigated the effects of Pcdha11 deficiency in mice. AmNA-modified anti-PCDHA11 ASOs significantly reduced the proliferation of gastric cancer cells and other solid tumors, whereas overexpression of PCDHA11 enhanced cell proliferation. The selected ASOs inhibited cellular functions related to the metastatic potential of gastric cancer cells, including migration, invasiveness, spheroid formation, and cancer stemness. Our findings revealed that AmNA-modified anti-PCDHA11 ASOs disrupted the AKT/mTOR, Wnt/β-catenin, and JAK/STAT signaling pathways. In mouse models of peritoneal metastasis (gastric and pancreatic cancer), systemic metastasis, and established subcutaneous tumors, administration of AmNA-modified anti-PCDHA11 ASOs inhibited tumor growth. ASO treatment induced reversible, dose- and sequence-dependent liver damage. Pcdha11-deficient mice demonstrated normal reproductive, organ, and motor functions. AmNA-modified anti-PCDHA11 ASOs offer a promising therapeutic strategy for the treatment of gastric cancer and other solid malignancies.
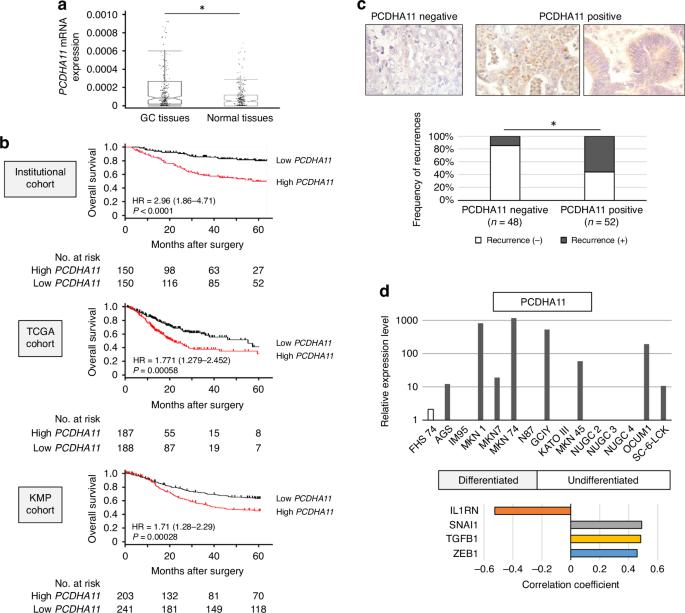
靶向致癌原粘连蛋白的双修饰反义寡核苷酸治疗胃癌。
研究背景本研究的目的是利用反义寡核苷酸(ASOs)靶向编码原粘连蛋白α11(PCDHA11)的基因,开发一种创新的治疗策略,并阐明PCDHA11在胃癌细胞中的作用:我们设计并筛选了54种氨基桥接核酸(AmNA)修饰的ASOs,根据PCDHA11的敲除效果、体外和体内活性以及脱靶效应筛选出这些ASOs。我们评估了AmNA修饰的抗PCDHA11 ASO对细胞功能和信号通路的影响,并研究了Pcdha11缺乏对小鼠的影响:结果:AmNA修饰的抗PCDHA11 ASOs能显著降低胃癌细胞和其他实体瘤的增殖,而过表达PCDHA11会增强细胞增殖。所选的 ASO 可抑制与胃癌细胞转移潜能有关的细胞功能,包括迁移、侵袭性、球形体形成和癌症干性。我们的研究结果表明,AmNA修饰的抗PCDHA11 ASO破坏了AKT/mTOR、Wnt/β-catenin和JAK/STAT信号通路。在小鼠腹膜转移(胃癌和胰腺癌)、全身转移和已形成的皮下肿瘤模型中,服用 AmNA 修饰的抗PCDHA11 ASO 可抑制肿瘤生长。ASO 治疗可诱导可逆的、剂量和序列依赖性肝损伤。Pcdha11缺陷小鼠的生殖、器官和运动功能正常:AmNA修饰的抗PCDHA11 ASO为治疗胃癌和其他实体恶性肿瘤提供了一种前景广阔的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

British Journal of Cancer
医学-肿瘤学
CiteScore
15.10
自引率
1.10%
发文量
383
审稿时长
6 months
期刊介绍:
The British Journal of Cancer is one of the most-cited general cancer journals, publishing significant advances in translational and clinical cancer research.It also publishes high-quality reviews and thought-provoking comment on all aspects of cancer prevention,diagnosis and treatment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


