Metformin reprograms tryptophan metabolism via gut microbiome-derived bile acid metabolites to ameliorate depression-Like behaviors in mice
IF 8.8
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
As an adjunct therapy, metformin enhances the efficacy of conventional antidepressant medications. However, its mode of action remains unclear. Here, metformin was found to ameliorate depression-like behaviors in mice exposed to chronic restraint stress (CRS) by normalizing the dysbiotic gut microbiome. Fecal transplants from metformin-treated mice ameliorated depressive behaviors in stressed mice. Microbiome profiling revealed that Akkermansia muciniphila (A. muciniphila), in particular, was markedly increased in the gut by metformin and that oral administration of this species alone was sufficient to reverse CRS-induced depressive behaviors and normalize aberrant stress-induced 5-hydroxytryptamine (5-HT) metabolism in the brain and gut. Untargeted metabolomic profiling further identified the bile acid metabolites taurocholate and deoxycholic acid as direct A. muciniphila-derived molecules that are, individually, sufficient to rescue the CRS-induced impaired 5-HT metabolism and depression-like behaviors. Thus, we report metformin reprograms 5-HT metabolism via microbiome-brain interactions to mitigate depressive syndromes, providing novel insights into gut microbiota-derived bile acids as potential therapeutic candidates for depressive mood disorders from bench to bedside.
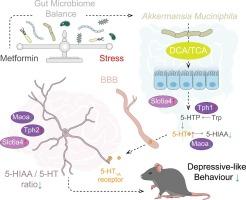
二甲双胍通过肠道微生物衍生的胆汁酸代谢物重编程色氨酸代谢,从而改善小鼠的抑郁样行为。
作为一种辅助疗法,二甲双胍可增强传统抗抑郁药物的疗效。然而,二甲双胍的作用模式仍不清楚。在这里,研究人员发现二甲双胍能通过使菌群失调的肠道微生物组正常化来改善长期暴露于束缚应激(CRS)的小鼠的抑郁样行为。二甲双胍处理过的小鼠的粪便移植可以改善应激小鼠的抑郁行为。微生物组图谱分析表明,二甲双胍能显著增加肠道中的Akkermansia muciniphila(A. muciniphila),而且仅口服该物种就足以逆转CRS诱导的抑郁行为,并使应激诱导的大脑和肠道中异常的5-羟色胺(5-HT)代谢正常化。非靶向代谢组学分析进一步确定了胆汁酸代谢物牛磺胆硷酸和脱氧胆硷酸是直接从粘蛋白蛛衍生的分子,它们足以单独挽救 CRS 诱导的 5-HT 代谢受损和抑郁样行为。因此,我们报告了二甲双胍通过微生物组与大脑的相互作用重编程 5-HT 代谢,从而缓解抑郁综合征,为肠道微生物组衍生的胆汁酸作为抑郁情绪障碍的潜在候选疗法提供了从实验到临床的新见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


