LSD1 is a promising target to treat cancers by modulating cell stemness
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
As the first discovered histone demethylase, LSD1 plays a vital role in maintaining pathological processes such as cancer, infection, and immune diseases. Based on previous researches, LSD1 is highly expressed in sorts of tumor cells such as acute myeloid leukemia, non-small cell lung cancer, prostate cancer, breast cancer and gastric cancer, etc. Therefore, targeting LSD1 is a prospective strategy for tumor treatment. Cancer stem cells could preserve self-renewal, cell proliferation, cell migration and malignant phenotype. So, the reduction of tumor cell stemness can effectively inhibit the growth of tumor cells, which may be a new strategy for the treatment of cancers. Up to now, there exist many researches confirming the significant role of LSD1 in regulating the stemness characteristics such as embryonic stem cells differentiation. Many reports show that inhibition of LSD1 effectively decreases the property of cancer cell stemness. However, there lacks a detailed review about the relationship between LSD1 and cancer cell stemness. Herein, in this review, we summarized the mechanisms how LSD1 regulates cell stemness comprehensively. In addition, some related inhibitors targeting LSD1 to reduce the proliferation characteristics of cancer stem cells are also described.
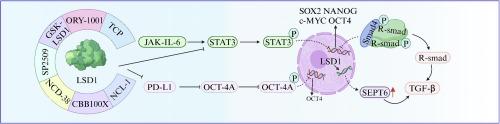
LSD1 是通过调节细胞干性来治疗癌症的一个很有前景的靶点。
作为第一个被发现的组蛋白去甲基化酶,LSD1 在维持癌症、感染和免疫疾病等病理过程中发挥着重要作用。根据以往的研究,LSD1 在各种肿瘤细胞中高表达,如急性髓性白血病、非小细胞肺癌、前列腺癌、乳腺癌和胃癌等。因此,靶向 LSD1 是一种治疗肿瘤的前瞻性策略。癌症干细胞可保持自我更新、细胞增殖、细胞迁移和恶性表型。因此,降低肿瘤细胞的干性可以有效抑制肿瘤细胞的生长,这可能是治疗癌症的一种新策略。迄今为止,已有许多研究证实,LSD1 在调控胚胎干细胞分化等干性特征方面发挥着重要作用。许多报告显示,抑制LSD1可有效降低癌细胞的干性特征。然而,目前还缺乏关于LSD1与癌细胞干性之间关系的详细综述。在这篇综述中,我们全面总结了LSD1调控细胞干性的机制。此外,还介绍了一些针对LSD1的相关抑制剂,以降低癌症干细胞的增殖特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


