GpsB Coordinates StkP Signaling as a PASTA Kinase Adaptor in Streptococcus pneumoniae Cell Division
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
StkP, the Ser/Thr protein kinase of the major human pathogen Streptococcus pneumoniae, monitors cell wall signals and regulates growth and division in response. In vivo, StkP interacts with GpsB, a cell division protein required for septal ring formation and closure, that affects StkP-dependent phosphorylation. Here, we report that although StkP has basal intrinsic kinase activity, GpsB promotes efficient autophosphorylation of StkP and phosphorylation of StkP substrates. Phosphoproteomic analyzes showed that GpsB is phosphorylated at several Ser and Thr residues. We confirmed that StkP directly phosphorylates GpsB in vitro and in vivo, with T79 and T83 being the major phosphorylation sites. In vitro, phosphoablative GpsB substitutions had a lower potential to stimulate StkP activity, whereas phosphomimetic substitutions were functional in terms of StkP activation. In vivo, substitutions of GpsB phosphoacceptor residues, either phosphoablative or mimetic, had a negative effect on GpsB function, resulting in reduced StkP-dependent phosphorylation and impaired cell division. The bacterial two-hybrid assay and co-immunoprecipitation of GpsB from cells with differentially active StkP indicated that increased phosphorylation of GpsB resulted in a more efficient interaction of GpsB with StkP. Our data suggest that GpsB acts as an adaptor that directly promotes StkP activity by mediating interactions within the StkP signaling hub, ensuring StkP recruitment into the complex and substrate specificity. We present a model that interaction of StkP with GpsB and its phosphorylation and dephosphorylation dynamically modulate kinase activity during exponential growth and under cell wall stress of S. pneumoniae, ensuring the proper functioning of the StkP signaling pathway.
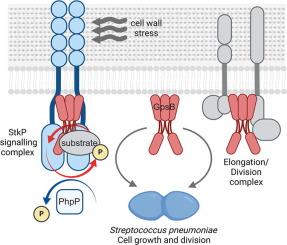
在肺炎链球菌细胞分裂过程中,GpsB 作为 PASTA 激酶适配器协调 StkP 信号传导。
StkP是人类主要病原体肺炎链球菌的Ser/Thr蛋白激酶,它能监测细胞壁信号并调节生长和分裂。在体内,StkP 与中隔环形成和闭合所需的细胞分裂蛋白 GpsB 相互作用,从而影响 StkP 依赖性磷酸化。在这里,我们报告说,虽然 StkP 具有基础固有激酶活性,但 GpsB 能促进 StkP 的有效自磷酸化和 StkP 底物的磷酸化。磷酸化蛋白组分析表明,GpsB 在多个 Ser 和 Thr 残基上被磷酸化。我们证实,StkP 在体外和体内直接磷酸化 GpsB,其中 T79 和 T83 是主要的磷酸化位点。在体外,GpsB的磷酸化取代对StkP活性的刺激潜力较低,而磷酸拟态取代对StkP的激活具有功能性。在体内,GpsB 磷酸化受体残基的置换,无论是磷酸化还是拟态,都会对 GpsB 的功能产生负面影响,导致 StkP 依赖性磷酸化减少和细胞分裂受损。细菌双杂交试验以及从具有不同活性 StkP 的细胞中对 GpsB 的共免疫沉淀表明,GpsB 磷酸化的增加导致 GpsB 与 StkP 的相互作用更有效。我们的数据表明,GpsB 是一种适配体,它通过介导 StkP 信号转导中枢内的相互作用直接促进 StkP 的活性,确保 StkP 被招募到复合物中并具有底物特异性。我们提出了一个模型,即 StkP 与 GpsB 的相互作用及其磷酸化和去磷酸化可动态调节肺炎双球菌在指数生长过程中和细胞壁应激状态下的激酶活性,从而确保 StkP 信号通路的正常运作。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


