Sulfa-Michael Addition on Dehydroalanine: A Versatile Reaction for Protein Modifications.
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Chemical modification of proteins has been widely applied in diagnostic and therapeutic processes. Here, we report a novel bioconjugation between sulfinic acids and amino acid dehydroalanine (Dha) in the context of both small molecules and proteins. This conjugation enables the rapid formation of sulfone linkages in a chemoselective and disulfide-compatible manner under biocompatible conditions with Dha residues chemically installed in proteins and thus provides a robust tool that is simple and has exquisite site selectivity for protein functionalization in a wide range.
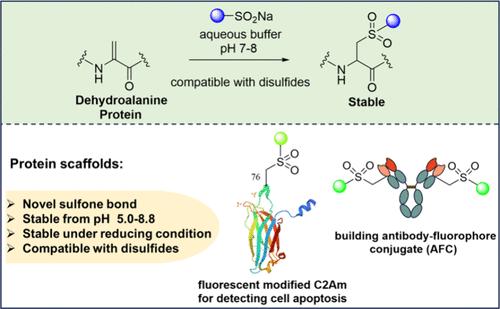
脱氢丙氨酸的磺胺-迈克尔加成:蛋白质修饰的多功能反应。
蛋白质的化学修饰已被广泛应用于诊断和治疗过程中。在此,我们报告了小分子和蛋白质中亚硫酸与氨基酸脱氢丙氨酸(Dha)之间的一种新型生物共轭。这种共轭方法能在生物相容的条件下,以化学选择性和二硫化物相容的方式与化学安装在蛋白质中的 Dha 残基快速形成砜基连接,从而为蛋白质的功能化提供了一种简单而又具有精湛的位点选择性的强大工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


