Photoredox/NHC Dual Catalysis Enabled de Novo Synthesis of α-Amino Acids Derivatives.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we report a mild and operationally simple photoredox/NHC dual catalysis strategy for the α-carboxylation of tertiary amine C(sp3)-H bonds using diethyl pyrocarbonate. This method offers a novel approach for synthesizing α-amino acid derivatives. The protocol features a broad substrate scope, accommodating both N-aryl tetrahydroisoquinolines (THIQ) and N-methyl aniline and is scalable to gram quantities. Additionally, it is suitable for the late-stage derivatization of certain pharmaceutical compounds.
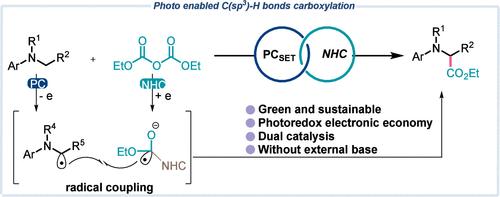
光氧化/NHC 双催化作用促进了 α-氨基酸衍生物的新合成。
在此,我们报告了一种温和且操作简单的光氧化/NHC 双催化策略,利用焦碳酸二乙酯对叔胺 C(sp3)-H 键进行α-羧化反应。该方法为合成 α 氨基酸衍生物提供了一种新方法。该方案的特点是底物范围广,可用于 N-芳基四氢异喹啉(THIQ)和 N-甲基苯胺,并可放大至克级数量。此外,它还适用于某些药物化合物的后期衍生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


