Modular Synthesis of α-Aryl Acrylamido Carboxylic Acids by Triple C-F Bond Cleavage of (Trifluoromethyl)alkenes with Unprotected Amino Acids.
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A straightforward and efficient strategy for the construction of tertiary and secondary α-aryl acrylamido carboxylic acids is reported. This N-acrylation protocol of unprotected amino acids is achieved by triple C-F bond cleavage of (trifluoromethyl)alkenes. This method features mild conditions, is operationally simple, is free of transition metals and racemization, can be used on a gram scale, and is compatible with various functional moieties. Mechanistic studies indicate that oxygen atom exchange happens among H2O, NaOH, and amino acids, and the oxygen atom of the amide moiety of the product is incorporated by the ipso-defluorooxylation of (trifluoromethyl)alkene.
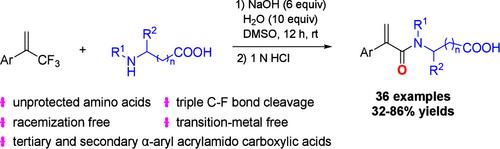
通过(三氟甲基)烯与未受保护的氨基酸的三重 C-F 键裂解,模块化合成 α-芳基丙烯酰胺基羧酸。
报告采用了一种直接高效的策略来构建三级和二级 α-芳基丙烯酰胺基羧酸。这种无保护氨基酸的 N-丙烯化协议是通过(三氟甲基)烯的三重 C-F 键裂解实现的。这种方法的特点是条件温和、操作简单、不含过渡金属和外消旋化,可在克级规模上使用,并与各种功能分子兼容。机理研究表明,氧原子交换发生在 H2O、NaOH 和氨基酸之间,产物中酰胺分子的氧原子通过(三氟甲基)烯的同氟氧 化作用结合在一起。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


