Coordinated, multicellular patterns of transcriptional variation that stratify patient cohorts are revealed by tensor decomposition
IF 41.7
1区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Tissue-level and organism-level biological processes often involve the coordinated action of multiple distinct cell types. The recent application of single-cell assays to many individuals should enable the study of how donor-level variation in one cell type is linked to that in other cell types. Here we introduce a computational approach called single-cell interpretable tensor decomposition (scITD) to identify common axes of interindividual variation by considering joint expression variation across multiple cell types. scITD combines expression matrices from each cell type into a higher-order matrix and factorizes the result using the Tucker tensor decomposition. Applying scITD to single-cell RNA-sequencing data on 115 persons with lupus and 83 persons with coronavirus disease 2019, we identify patterns of coordinated cellular activity linked to disease severity and specific phenotypes, such as lupus nephritis. scITD results also implicate specific signaling pathways likely mediating coordination between cell types. Overall, scITD offers a tool for understanding the covariation of cell states across individuals, which can yield insights into the complex processes that define and stratify disease. Unsupervised analysis of single-cell RNA-sequencing data across cell types and individuals predicts disease severity.

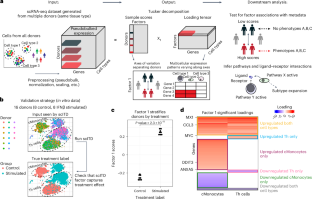
张量分解法揭示了使患者群体分层的多细胞转录变异协调模式
组织层面和生物体层面的生物过程往往涉及多种不同细胞类型的协调作用。最近对许多个体进行的单细胞检测有助于研究一种细胞类型的供体水平变异与其他细胞类型的供体水平变异之间的联系。在这里,我们介绍了一种名为单细胞可解释张量分解(scITD)的计算方法,通过考虑多种细胞类型的联合表达变异来确定个体间变异的共同轴线。scITD将每种细胞类型的表达矩阵组合成一个高阶矩阵,并使用塔克张量分解法对结果进行因子化。将 scITD 应用于 115 名红斑狼疮患者和 83 名冠状病毒患者的单细胞 RNA 序列数据,我们确定了与疾病严重程度和特定表型(如狼疮肾炎)相关的细胞协调活动模式。总之,scITD 为了解不同个体细胞状态的共变性提供了一种工具,它可以帮助我们深入了解定义和分层疾病的复杂过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature biotechnology
工程技术-生物工程与应用微生物
CiteScore
63.00
自引率
1.70%
发文量
382
审稿时长
3 months
期刊介绍:
Nature Biotechnology is a monthly journal that focuses on the science and business of biotechnology. It covers a wide range of topics including technology/methodology advancements in the biological, biomedical, agricultural, and environmental sciences. The journal also explores the commercial, political, ethical, legal, and societal aspects of this research.
The journal serves researchers by providing peer-reviewed research papers in the field of biotechnology. It also serves the business community by delivering news about research developments. This approach ensures that both the scientific and business communities are well-informed and able to stay up-to-date on the latest advancements and opportunities in the field.
Some key areas of interest in which the journal actively seeks research papers include molecular engineering of nucleic acids and proteins, molecular therapy, large-scale biology, computational biology, regenerative medicine, imaging technology, analytical biotechnology, applied immunology, food and agricultural biotechnology, and environmental biotechnology.
In summary, Nature Biotechnology is a comprehensive journal that covers both the scientific and business aspects of biotechnology. It strives to provide researchers with valuable research papers and news while also delivering important scientific advancements to the business community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


