Loss of electrical β-cell to δ-cell coupling underlies impaired hypoglycaemia-induced glucagon secretion in type-1 diabetes
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
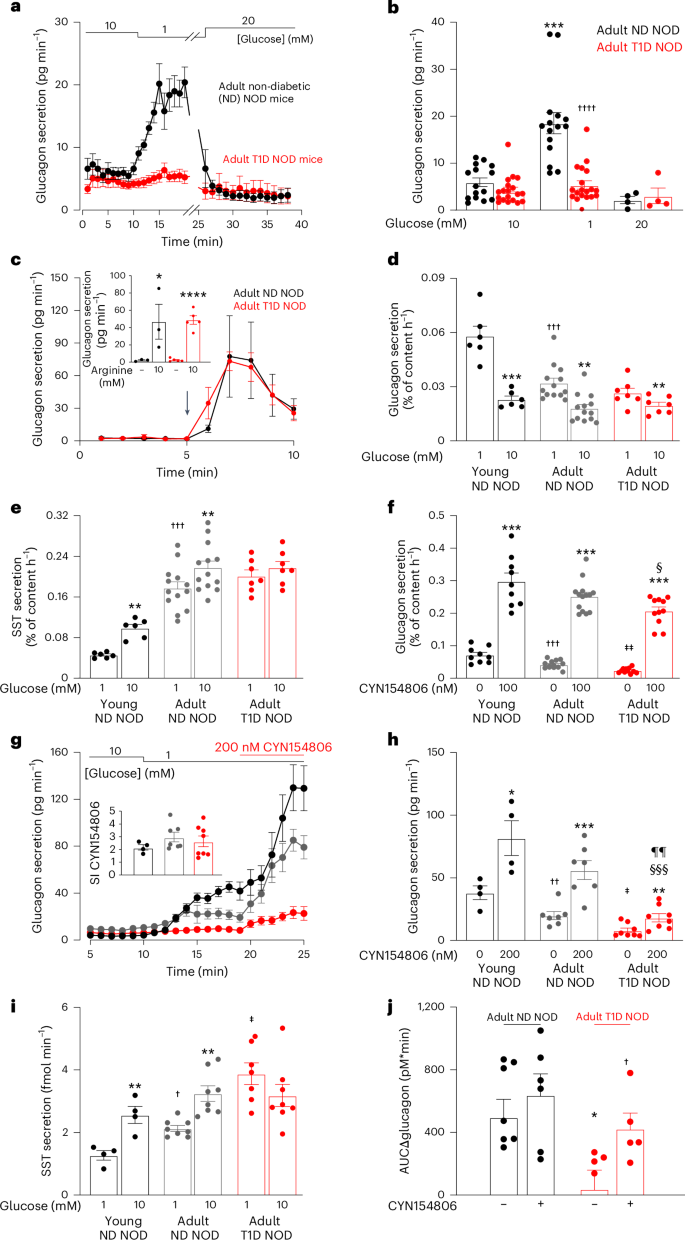
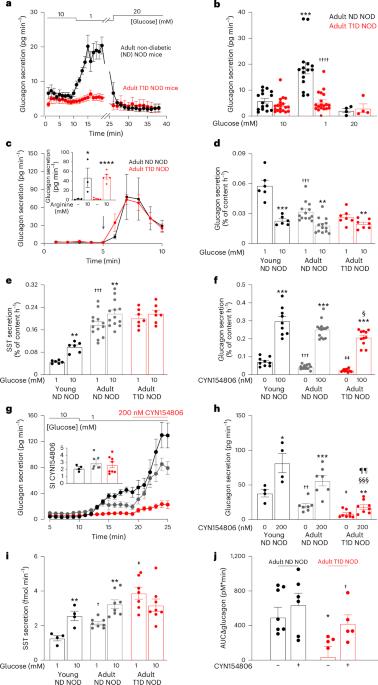
Diabetes mellitus involves both insufficient insulin secretion and dysregulation of glucagon secretion1. In healthy people, a fall in plasma glucose stimulates glucagon release and thereby increases counter-regulatory hepatic glucose production. This response is absent in many patients with type-1 diabetes (T1D)2, which predisposes to severe hypoglycaemia that may be fatal and accounts for up to 10% of the mortality in patients with T1D3. In rats with chemically induced or autoimmune diabetes, counter-regulatory glucagon secretion can be restored by SSTR antagonists4–7 but both the underlying cellular mechanism and whether it can be extended to humans remain unestablished. Here, we show that glucagon secretion is not stimulated by low glucose in isolated human islets from donors with T1D, a defect recapitulated in non-obese diabetic mice with T1D. This occurs because of hypersecretion of somatostatin, leading to aberrant paracrine inhibition of glucagon secretion. Normally, KATP channel-dependent hyperpolarization of β-cells at low glucose extends into the δ-cells through gap junctions, culminating in suppression of action potential firing and inhibition of somatostatin secretion. This ‘electric brake’ is lost following autoimmune destruction of the β-cells, resulting in impaired counter-regulation. This scenario accounts for the clinical observation that residual β-cell function correlates with reduced hypoglycaemia risk8. Impaired somatostatin secretion owing to loss of β- to δ-cell electrical coupling underlies defective counter-regulatory glucagon secretion in type-1 diabetes.
1 型糖尿病患者低血糖诱导的胰高血糖素分泌受损的原因是 β 细胞与 δ 细胞间电偶联的缺失
糖尿病既有胰岛素分泌不足的问题,也有胰高血糖素分泌失调的问题1。在健康人体内,血浆葡萄糖的下降会刺激胰高血糖素的释放,从而增加反调节的肝糖分泌。许多 1 型糖尿病(T1D)患者2 都没有这种反应,这容易导致严重的低血糖症,而低血糖症可能是致命的,占 T1D 患者死亡率的 10%3。在化学诱导或自身免疫性糖尿病大鼠中,SSTR 拮抗剂可恢复反调节性胰高血糖素分泌4,5,6,7,但其潜在的细胞机制以及是否可推广至人类仍未确定。在这里,我们发现,在来自 T1D 供体的离体人胰岛中,低血糖不会刺激胰高血糖素分泌,这一缺陷在患有 T1D 的非肥胖糖尿病小鼠中得到了重现。出现这种情况是因为体生长抑素分泌过多,导致对胰高血糖素分泌的旁分泌抑制失常。正常情况下,β 细胞在低糖时依靠 KATP 通道的超极化作用通过间隙连接延伸到 δ 细胞,最终抑制动作电位的发射并抑制体生长激素的分泌。这种 "电子制动器 "会在β细胞被自身免疫性破坏后消失,从而导致反调节功能受损。临床观察发现,残余的 β 细胞功能与低血糖风险降低相关,这就是这种情况的原因8。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


