Unlocking Lattice Oxygen on Selenide-Derived NiCoOOH for Amine Electrooxidation and Efficient Hydrogen Production
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
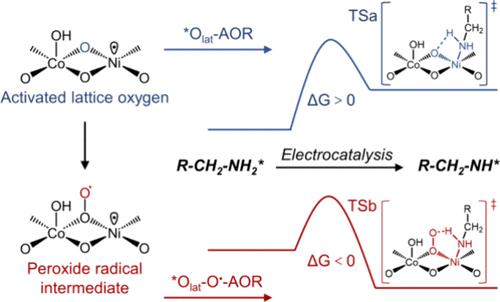
In pursuit of advancing the electrooxidation of amines, which is typically encumbered by the inertness of C(sp3)–H/N(sp3)–H bonds, our study introduces a high-performance electrocatalyst that significantly enhances the production efficiency of vital chemicals and fuels. We propose a novel electrocatalytic strategy employing a uniquely designed (NixCo1–x)Se2-R electrocatalyst, which is activated through Se–O exchange and electron orbital spin manipulation. This catalyst efficiently generates M4+ species, thus enabling the activation of lattice oxygen and streamlining the electrooxidation of amines. Empirical evidence from isotope labeling, molecular probes, and computational analyses indicates that the electrocatalyst fosters the formation of energetically favorable peroxy radical intermediates, which substantially expedite the reaction kinetics. The refined electrocatalyst achieves an exceptional current density of 20 mA cm–2 at a potential of 1.315 V, with selectivity surpassing 99% for propionitrile, while demonstrating remarkable stability over 560 h. This work emphasizes the criticality of deciphering the fundamental mechanisms of amine electrooxidation and charts a more sustainable pathway for the nitrile and hydrogen production, marking a substantial advancement in the field of electrocatalysis.
释放硒化镍钴氧化物上的晶格氧,实现胺电氧化和高效制氢
胺的电氧化通常受到 C(sp3)-H/N(sp3)-H 键惰性的限制,为了推进胺的电氧化,我们的研究引入了一种高性能电催化剂,可显著提高重要化学品和燃料的生产效率。我们提出了一种新颖的电催化策略,即采用独特设计的(NixCo1-x)Se2-R 电催化剂,通过 Se-O 交换和电子轨道自旋操作激活该催化剂。这种催化剂能有效生成 M4+ 物种,从而激活晶格氧并简化胺的电氧化过程。来自同位素标记、分子探针和计算分析的经验证据表明,这种电催化剂促进了能量上有利的过氧自由基中间体的形成,从而大大加快了反应动力学。改进后的电催化剂在 1.315 V 的电位下可达到 20 mA cm-2 的超大电流密度,对丙腈的选择性超过 99%,同时在 560 小时内表现出显著的稳定性。这项工作强调了破译胺电氧化基本机制的重要性,并为腈和氢的生产描绘了一条更可持续的途径,标志着电催化领域的重大进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


