When van der Waals Met Kagome: A 2D Antimonide with a Vanadium-Kagome Network
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
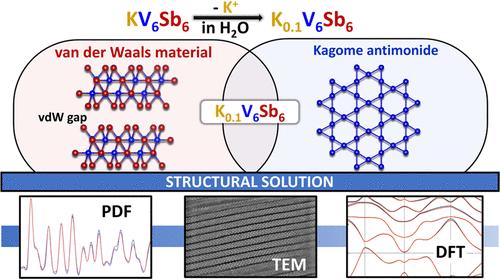
2D materials showcase unconventional properties emerging from quantum confinement effects. In this work, a “soft chemical” route allows for the deintercalation of K+ from the layered antimonide KV6Sb6, resulting in the discovery of a new metastable 2D-Kagome antimonide K0.1(1)V6Sb6 with a van der Waals gap of 3.2 Å. The structure of K0.1(1)V6Sb6 was determined via the synergistic techniques, including X-ray pair distribution function analysis, advanced transmission electron microscopy, and density functional theory calculations. The K0.1(1)V6Sb6 compound crystallizes in the monoclinic space group C2/m (a = 9.57(2) Å, b = 5.502(8) Å, c = 10.23(2) Å, β = 97.6(2)°, Z = 2). The [V6Sb6] layers in K0.1(1)V6Sb6 are retained upon deintercalation and closely resemble the layers in the parent compound, yet deintercalation results in a relative shift of the adjacent [V6Sb6] layers. The magnetic properties of the K0.1(1)V6Sb6 phase in the 2–300 K range are comparable to those of KV6Sb6 and another Kagome antimonide KV3Sb5, consistent with nearly temperature-independent paramagnetism. Electronic band structure calculation suggests a nontrivial band topology with flat bands and opening of band crossing afforded by deintercalation. Transport property measurements reveal a metallic nature for K0.1(1)V6Sb6 and a low thermal conductivity of 0.6 W K–1 m–1 at 300 K. Additionally, ion exchange in KV6Sb6 via a solvothermal route leads to a successful partial exchange of K+ with A+ (A = Na, Rb, and Cs). This study highlights the tunability of the layered structure of the KV6Sb6 compound, providing a rich playground for the realization of new 2D materials.
当范德瓦耳斯遇到鹿目:具有钒-鹿目网络的二维锑化物
二维材料展示了量子约束效应所产生的非常规特性。在这项研究中,通过 "软化学 "途径,K+得以从层状锑化物KV6Sb6中脱插,从而发现了一种新的可蜕变二维-可果美的锑化物K0.1(1)V6Sb6,其范德华间隙为3.2埃。K0.1(1)V6Sb6的结构是通过X射线对分布函数分析、先进的透射电子显微镜和密度泛函理论计算等协同技术确定的。K0.1(1)V6Sb6 化合物在单斜空间群 C2/m 中结晶(a = 9.57(2) Å, b = 5.502(8) Å, c = 10.23(2) Å, β = 97.6(2)°, Z = 2)。脱插后,K0.1(1)V6Sb6 中的[V6Sb6]层被保留下来,与母体化合物中的[V6Sb6]层非常相似,但脱插会导致相邻的[V6Sb6]层发生相对移动。K0.1(1)V6Sb6 相在 2-300 K 范围内的磁性能与 KV6Sb6 和另一种 Kagome锑化物 KV3Sb5 相当,这与几乎与温度无关的顺磁性相一致。电子能带结构计算表明,该物质的能带拓扑结构并不复杂,具有平坦的能带,并能通过去插层打开能带交叉。此外,通过溶热途径在 KV6Sb6 中进行离子交换,成功地实现了 K+与 A+(A = Na、Rb 和 Cs)的部分交换。这项研究凸显了 KV6Sb6 化合物层状结构的可调性,为实现新型二维材料提供了丰富的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


