Ligand-Controlled Regiodivergent Ring Expansion of Benzosilacyclobutenes with Alkynes en Route to Axially Chiral Silacyclohexenyl Arenes
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A ligand-controlled regiodivergent and enantioselective ring expansion of benzosilacyclobutenes with internal naphthyl alkynes has been achieved by adjusting the ligand cavity size. The ligand (S)-8H-binaphthyl phosphoramidite, featuring small methyl groups on its arms, provides a spacious cavity that favors sterically demanding Si–Csp3 ring expansion, predominantly yielding axially chiral (S)-1-silacyclohexenyl arenes. In contrast, the ligand (R)-spiro phosphoramidite, with bulky t-Bu groups on its arms, offers a compact cavity that facilitates less sterically demanding Si–Csp2 ring expansion, leading primarily to axially chiral (S)-2-silacyclohexenyl arenes. Density functional theory calculations delineate distinct mechanistic pathways for each ring expansion route and elucidate their regio- and enantioselectivity.
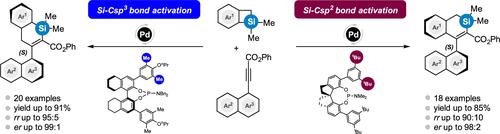
通过配体控制苯并硅杂环丁烯与炔烃的歧环扩展,制备轴向手性硅杂环己烯烯环
通过调整配体空腔的大小,实现了具有内部萘基烷炔的苯并硅杂环丁烯的配体控制的变异性和对映体选择性扩环。配体(S)-8H-联萘亚磷酰胺的臂上带有小甲基,提供了一个宽敞的空腔,有利于立体要求较高的 Si-Csp3 扩环,主要产生轴向手性的(S)-1-硅杂环己烯炔。相反,配体 (R)-spiro phosphoramidite 的臂上含有大量 t-Bu 基团,提供了一个紧凑的空腔,有利于立体要求较低的 Si-Csp2 环扩展,主要产生轴向手性 (S)-2 硅杂环己烯炔。密度泛函理论计算为每种扩环途径划定了不同的机理途径,并阐明了它们的区域选择性和对映体选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


