Iodine-Promoted Cascade Redox Cyclization to Access 2-Arylbenzothiazoles Using Elemental Sulfur
IF 2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A straightforward and efficient method has been developed to access a variety of benzothiazole derivatives via cascade reductive annulation. Iodine mediated, one-pot three-component reaction of o-chloronitroarenes, aryl aldehydes, and elemental sulfur effectively produce benzothiazoles. Moreover, the metal-free strategy allows the facile synthesis of diverse 2-substituted benzothiazoles through multiple carbon–heteroatom bonds in good yields. The present protocol features a greener approach, readily accessible reagents, broad substrate scope, high product yields, and operational simplicity.
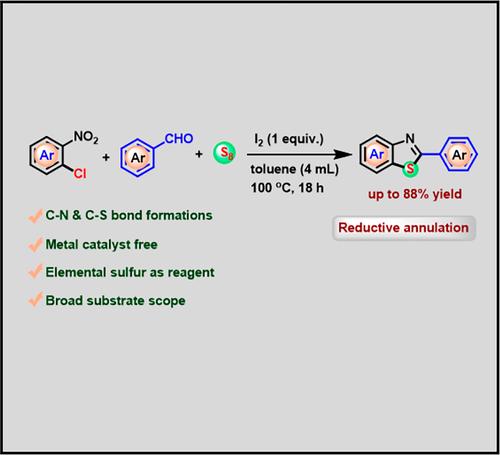
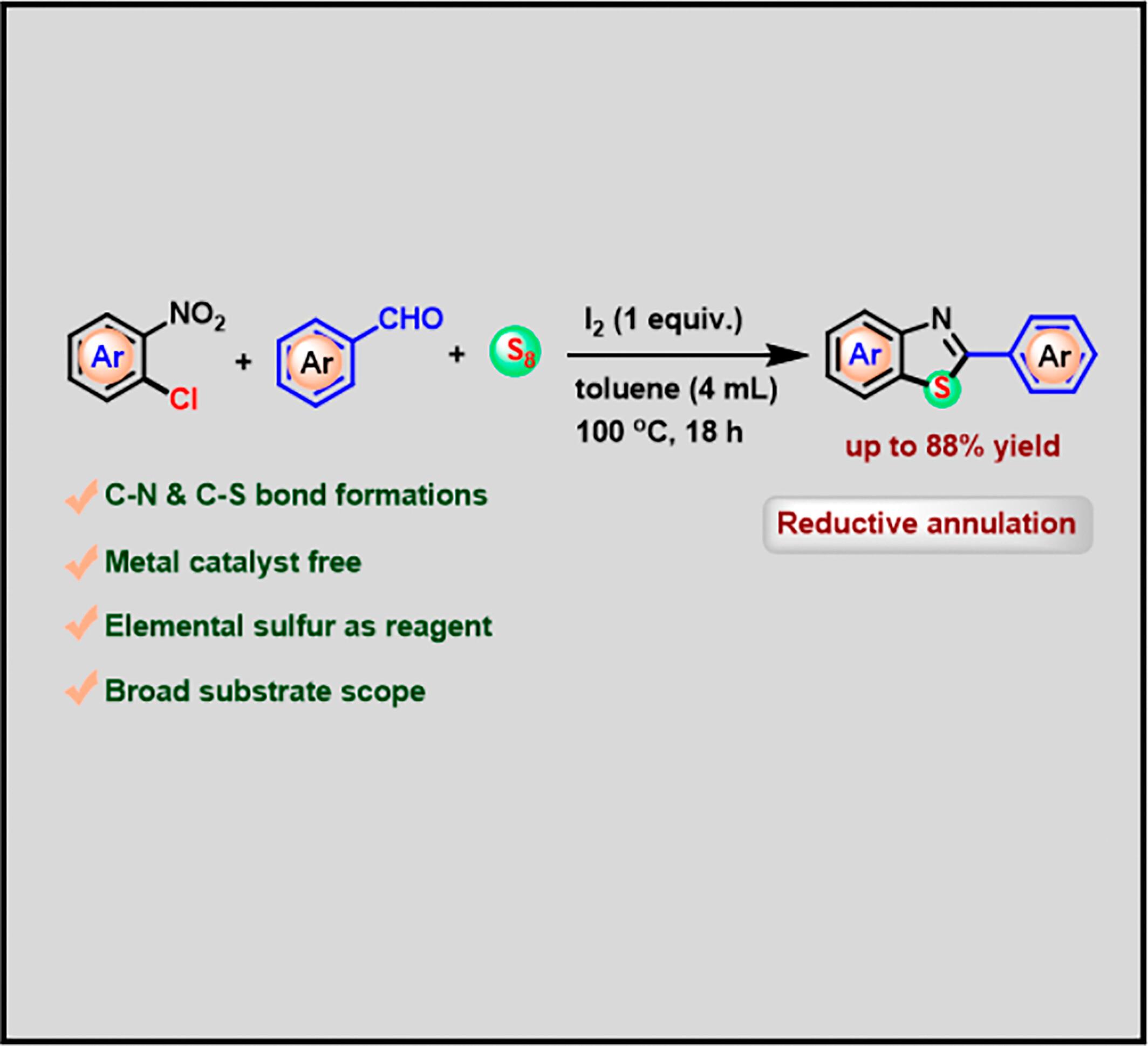
利用元素硫进行碘促进级联氧化还原环化以获得 2-芳基苯并噻唑
通过级联还原环化反应获得多种苯并噻唑衍生物的方法简单而高效。碘介导的邻氯硝基苯醚、芳基醛和元素硫的单锅三组分反应有效地生成了苯并噻唑。此外,这种无金属策略还能通过多个碳-杂原子键以良好的产率轻松合成多种 2-取代的苯并噻唑。本方案具有更环保的方法、容易获得的试剂、广泛的底物范围、高产率和操作简单等特点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.20
自引率
4.20%
发文量
177
审稿时长
3.9 months
期刊介绍:
The Journal of Heterocyclic Chemistry is interested in publishing research on all aspects of heterocyclic chemistry, especially development and application of efficient synthetic methodologies and strategies for the synthesis of various heterocyclic compounds. In addition, Journal of Heterocyclic Chemistry promotes research in other areas that contribute to heterocyclic synthesis/application, such as synthesis design, reaction techniques, flow chemistry and continuous processing, multiphase catalysis, green chemistry, catalyst immobilization and recycling.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


