Signaling Mechanism of Cuproptosis Activating cGAS-STING Immune Pathway
引用次数: 0
Abstract
Copper-mediated programmed cell death, which influences the regulation of tumor progression, is an effective approach for antitumor molecular therapy. Unlike apoptosis, copper complex-induced cuproptosis by lipid-acylated protein aggregation triggers the mitochondrial proteotoxic stress response, which could be associated with immunomodulation. However, it remains a great challenge to understand the distinctive molecular mechanisms that presumably activate immunity by cuproptosis. Here, the new nonlabeling fluorescent molecular tools of Cu-DPPZ-Py+ and Cu-DPPZ-Ph are synthesized and used to investigate the differential immune signaling mechanisms induced by copper-mediated cuproptosis or apoptosis. With Cu-DPPZ-Py+ and Cu-Elesclomol, there is strong evidence that the triggering cuproptosis significantly drives mitochondrial DNA (mtDNA) release to activate innate immunity via cyclic GMP-AMP synthase-stimulation of interferon genes (cGAS-STING), which can improve T cell antitumor immunity in vivo. By contrast, it is observed that Cu-DPPZ-Ph treated tumor cells could release intracellular caspase-3, resulting in apoptosis-associated immunosuppression. This study supports insights into how cuproptosis bridges cGAS-STING immune pathways, contributing to the development of cuproptosis-based antitumor immunotherapy.
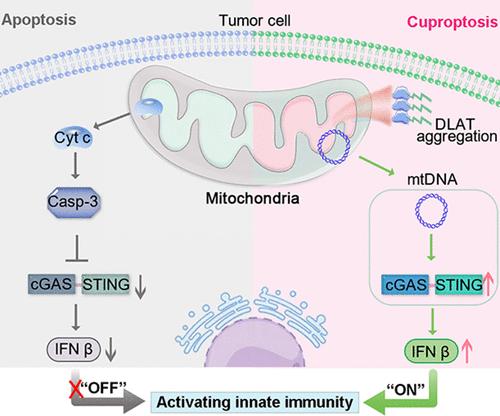
杯突激活 cGAS-STING 免疫途径的信号机制
铜介导的细胞程序性死亡影响着肿瘤的进展调控,是抗肿瘤分子治疗的一种有效方法。与细胞凋亡不同,铜复合物通过脂质酰化蛋白聚集诱导的铜中毒会引发线粒体蛋白毒性应激反应,这可能与免疫调节有关。然而,了解杯突激活免疫的独特分子机制仍然是一个巨大的挑战。本文合成了 Cu-DPPZ-Py+ 和 Cu-DPPZ-Ph 这两种新型非标记荧光分子工具,并将其用于研究铜介导的杯突症或细胞凋亡诱导的不同免疫信号转导机制。有确凿证据表明,Cu-DPPZ-Py+和Cu-Elesclomol诱导的杯突症能显著推动线粒体DNA(mtDNA)的释放,通过环GMP-AMP合成酶刺激干扰素基因(cGAS-STING)激活先天性免疫,从而提高体内T细胞的抗肿瘤免疫力。相比之下,Cu-DPPZ-Ph 处理的肿瘤细胞可释放细胞内的 caspase-3,从而导致与细胞凋亡相关的免疫抑制。这项研究有助于深入了解杯突如何连接 cGAS-STING 免疫途径,从而促进基于杯突的抗肿瘤免疫疗法的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


